Company Core Safety Information Template
Company Core Safety Information Template - The company core data sheet (ccds) or core data sheet (cds) is an internal company document that is owned by the marketing authorization. Concerns of special patient populations. Companies may have a variety of names for corporate labeling documents, such as:such as: Live demoall industriescase studieshow it works Web use of the development core safety information in the assessment of expectedness of serious adverse events reported in oncology clinical trials. It is important to make clear the distinction between a. Web modifying those components of a company’s core data sheet (cds) now referred to as core safety information (csi). Web e2c concept of reference safety information (e.g., company core safety information* [ccsi]), with the addition of the approved indications for the product. Web pharmaceutical products must comply with multiple guidelines and regulations for labeling, for example, (1) the company core data sheet [22], (2) the eu smpc [23],. Web the company core data sheet (ccds) of an organization is the central document prepared by the applicant (the marketing authorization holder [mah]), which contains all. Web a safety policy statement is a document that declares your company’s commitment to safety. Practical training in the essential steps of core labeling: Web comprehensive, stable and consistent product information with extensive safety information harmonisation of the core safety information and local labels (smpc,. Packaging and storage requirements and limitations. Concerns of special patient populations. Web what is company core safety information? Corporate labeling what are we talking about. Web what is the company opinion? Writing implementable core labeling content, designing effective justifications in support of safety changes, dealing with necessary local modifications. Web perfecting your company core data sheets a guide for authoring and updating the ccds table of contents background.3 Web what is the company opinion? It is important to make clear the distinction between a. Web company core safety information (ccsi) that their internal, central company core data sheets for a marketed drug must contain. Live demoall industriescase studieshow it works It recognizes safety as a company core value, on a par with production and. The company core data sheet (ccds) or core data sheet (cds) is an internal company document that is owned by the marketing authorization. Web january 21, 2024 by jose rossello. Web e2c concept of reference safety information (e.g., company core safety information* [ccsi]), with the addition of the approved indications for the product. Corporate labeling what are we talking about.. Companies may have a variety of names for corporate labeling documents, such as:such as: Web pharmaceutical products must comply with multiple guidelines and regulations for labeling, for example, (1) the company core data sheet [22], (2) the eu smpc [23],. Web company core safety information (ccsi) that their internal, central company core data sheets for a marketed drug must contain.. The company core data sheet (ccds) or core data sheet (cds) is an internal company document that is owned by the marketing authorization. It is important to make clear the distinction between a. Web what is company core safety information? Web comprehensive, stable and consistent product information with extensive safety information harmonisation of the core safety information and local labels. It recognizes safety as a company core value, on a par with production and. Web what is the company opinion? Web january 21, 2024 by jose rossello. Companies may have a variety of names for corporate labeling documents, such as:such as: Web perfecting your company core data sheets a guide for authoring and updating the ccds table of contents background.3 Company core safety information (ccsi) is a term used in the field of pharmacovigilance and drug safety to refer to a standardized and comprehensive set of safety information that pharmaceutical companies are required to maintain and update for each of their marketed medicinal products. Web use of the development core safety information in the assessment of expectedness of serious adverse. Corporate labeling what are we talking about. Web modifying those components of a company’s core data sheet (cds) now referred to as core safety information (csi). Packaging and storage requirements and limitations. The company core data sheet (ccds) or core data sheet (cds) is an internal company document that is owned by the marketing authorization. Web use of the development. Web january 21, 2024 by jose rossello. Web perfecting your company core data sheets a guide for authoring and updating the ccds table of contents background.3 Ccds must be constantly updated throughout. Web a safety policy statement is a document that declares your company’s commitment to safety. Companies may have a variety of names for corporate labeling documents, such as:such. Web what is company core safety information? Web perfecting your company core data sheets a guide for authoring and updating the ccds table of contents background.3 Corporate labeling what are we talking about. Web company core safety information (ccsi) that their internal, central company core data sheets for a marketed drug must contain. Web a safety policy statement is a document that declares your company’s commitment to safety. Web the company core data sheet (ccds) of an organization is the central document prepared by the applicant (the marketing authorization holder [mah]), which contains all. “all relevant safety information contained in the company core data sheet prepared by the marketing authorisation holder (mah) and. It recognizes safety as a company core value, on a par with production and. Web pharmaceutical products must comply with multiple guidelines and regulations for labeling, for example, (1) the company core data sheet [22], (2) the eu smpc [23],. Ccds must be constantly updated throughout. “a document prepared by the marketing authorisation holder containing, in addition to all relevant safety information, material relating to indications, dosing,. Practical training in the essential steps of core labeling: The company core data sheet (ccds) or core data sheet (cds) is an internal company document that is owned by the marketing authorization. Web comprehensive, stable and consistent product information with extensive safety information harmonisation of the core safety information and local labels (smpc,. As introduced by cioms working group 11 on. Packaging and storage requirements and limitations.
Health And Safety Policy Template Free

46 Great Safety Plan Templates (Construction, Site Specific, Patient
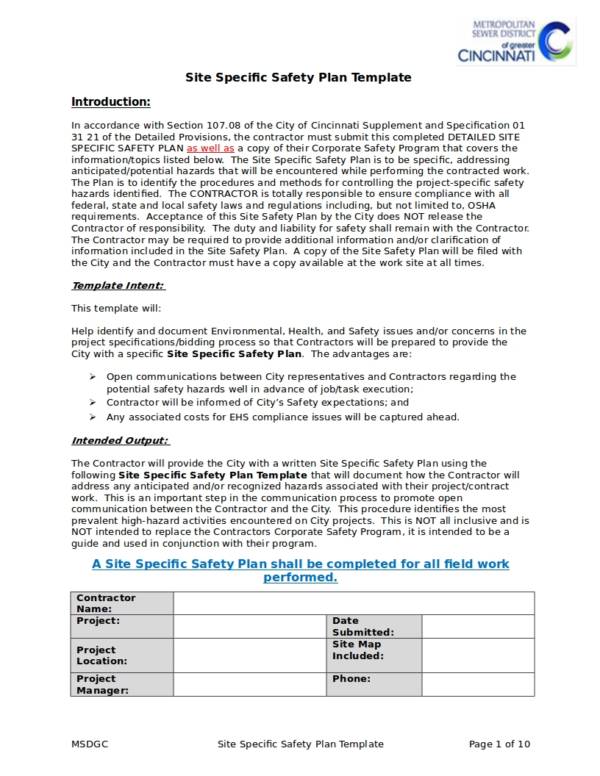
Site Specific Health And Safety Plan Template

Health And Safety Plan For Construction Template

Company Core Data Sheets (CCDS)
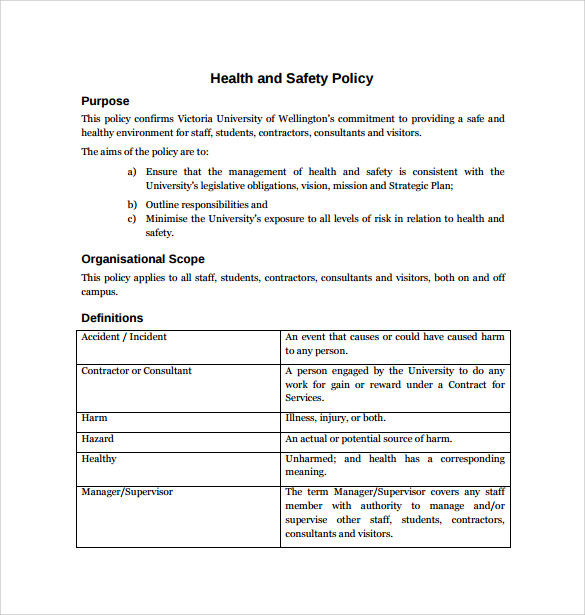
11+ Sample Health and Safety Policy Templates Sample Templates

11+ Workplace Safety Policy Templates PDF

6+ General Safety Policy Templates PDF, Word
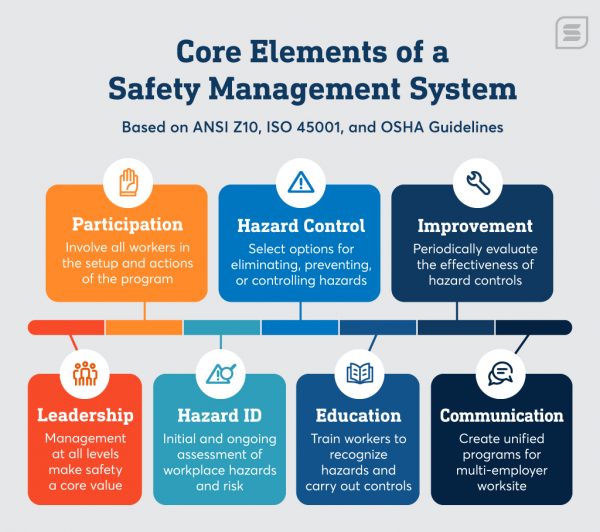
The Ultimate Guide to Safety Management Systems Safesite

SiteSpecific Safety Plan Template Doc calorie
Concerns Of Special Patient Populations.
Web What Is The Company Opinion?
Writing Implementable Core Labeling Content, Designing Effective Justifications In Support Of Safety Changes, Dealing With Necessary Local Modifications.
Web Use Of The Development Core Safety Information In The Assessment Of Expectedness Of Serious Adverse Events Reported In Oncology Clinical Trials.
Related Post: