Combustion Reaction Examples Drawing
Combustion Reaction Examples Drawing - Browning happens when proteins react with reducing sugars through high heat, resulting in a golden. What is this substance in the air that a candle needs to burn? Drawing energy level diagrams of the combustion of methane. Ch 4 (g) + 2 o 2 (g) → co 2 (g) + 2 h 2 o (g) burning of naphthalene. The formula for ethanol is \(\ce{c_2h_5oh}\). Ethene (c2h4) + oxygen æ. Write the balanced chemical equation that shows the combustion of ethanol. A given chemical reaction can be represented using a particulate diagram, in which the reaction mixture is depicted both before the reaction occurs and after the reaction has proceeded completely as possible. C 10 h 8 + 12 o 2 → 10 co 2 + 4 h 2 o. Combustion reactions are always exothermic (δ h is negative) so the reactants should be drawn higher in energy than the products. A given chemical reaction can be represented using a particulate diagram, in which the reaction mixture is depicted both before the reaction occurs and after the reaction has proceeded completely as possible. 2 c 2 h 6 + 7 o 2 → 4 co 2 + 6 h 2 o. C 2 h 5 oh ( l) + o 2. Enthalpy of reaction, formation, and combustion. C x h y + (x+y/4) o 2 → x co 2 + (y/2) h 2 o. There are a few examples of combustion reactions of metals, nonmetals, and hydrocarbons. Combustion is a reaction in which a substance reacts with oxygen gas. Ethanol can be used as a fuel source in an alcohol lamp. Ch4 (g) + 2o2 (g) → co2 (g) + 2h2o (l) step 2: Web butane (c4h10) + oxygen æ. They are changing into different molecules by reacting with a substance in the air. Combustion of butane (commonly found in lighters) Web the process of burning (as opposed to evaporating) is a chemical reaction, a chemical change. This is a composition reaction. C 2 h 5 oh (l) + o 2 (g) → The chemical equation for the complete combustion of methane is: C 10 h 8 + 12 o 2 → 10 co 2 + 4 h 2 o. C 2 h 5 oh ( l) + o 2 ( g) → co 2 ( g). Complete and balance each combustion equation. The combustion of lpg fuel in gas stoves for the cooking of food involves a combustion reaction between the oxygen present in the atmosphere and the liquefied petroleum gas. C x h y + (x+y/4) o 2 → x co 2 + (y/2) h 2 o. Write the balanced chemical equation that shows the. Web drawing particulate models of reaction mixtures (video) | khan academy. C 2 h 5 oh ( l) + o 2 ( g) → co 2 ( g) + h 2 o ( g) balance the equation. C x h y + (x+y/4) o 2 → x co 2 + (y/2) h 2 o. Web we can also use the. Combustion of butane (commonly found in lighters) Hydrocarbons react with oxygen to form carbon dioxide and water. Ch4 (g) + 2o2 (g) → co2 (g) + 2h2o (l) step 2: Enthalpy of reaction, formation, and combustion. Combustion reactions are always exothermic (δ h is negative) so the reactants should be drawn higher in energy than the products. Web here are examples of balanced equations for combustion reactions: The combustion of lpg fuel in gas stoves for the cooking of food involves a combustion reaction between the oxygen present in the atmosphere and the liquefied petroleum gas. C 2 h 5 oh (l) + o 2 (g) → Write the balanced equation for the combustion of ethanol. Ch4. Web combustion reactions are a type of redox reaction. C x h y + (x+y/4) o 2 → x co 2 + (y/2) h 2 o. The combustion of lpg fuel in gas stoves for the cooking of food involves a combustion reaction between the oxygen present in the atmosphere and the liquefied petroleum gas. A given chemical reaction can. Browning happens when proteins react with reducing sugars through high heat, resulting in a golden. They are changing into different molecules by reacting with a substance in the air. Ch4 (g) + 2o2 (g) → co2 (g) + 2h2o (l) step 2: C 3 h 8 (g) + 5 o 2 (g) → 3 co 2 (g) + 4 h. C 2 h 5 oh ( l) + 3 o 2 ( g) → 2 co 2 ( g) + 3 h 2 o ( g) There are a few examples of combustion reactions of metals, nonmetals, and hydrocarbons. Ethene (c2h4) + oxygen æ. Here, x = 3 and y = 8. Ethyne (c2h2) + oxygen æ. Illustrate the presence of water and carbon dioxide in the products of hydrocarbon combustion in this demonstration. Hexane (c6h14) + oxygen æ. C 2 h 5 oh (l) + o 2 (g) → Web we can also use the general equation to balance the combustion reaction equation. A simplified diagram of a combustion reaction where oxygen is the oxidant. The products of the reaction are co 2 and h 2 o, so our unbalanced equation is \[\ce{c3h8 + o2 → co2 + h2o} \nonumber \] Combustion reactions are always exothermic (δ h is negative) so the reactants should be drawn higher in energy than the products. Web here are examples of balanced equations for combustion reactions: C 10 h 8 + 12 o 2 → 10 co 2 + 4 h 2 o. When a match is struck, friction heats the head to a temperature at which the chemicals react and generate more heat than can escape into the air, and they burn with a flame. Combustion reactions are always exothermic (δ h is negative) so the reactants should be drawn higher in energy than the products.
What is Combustion? YouTube

Combustion reaction, illustration Stock Image F027/1851 Science

Chemical Reaction Definition, Types and Examples Class 10 Science
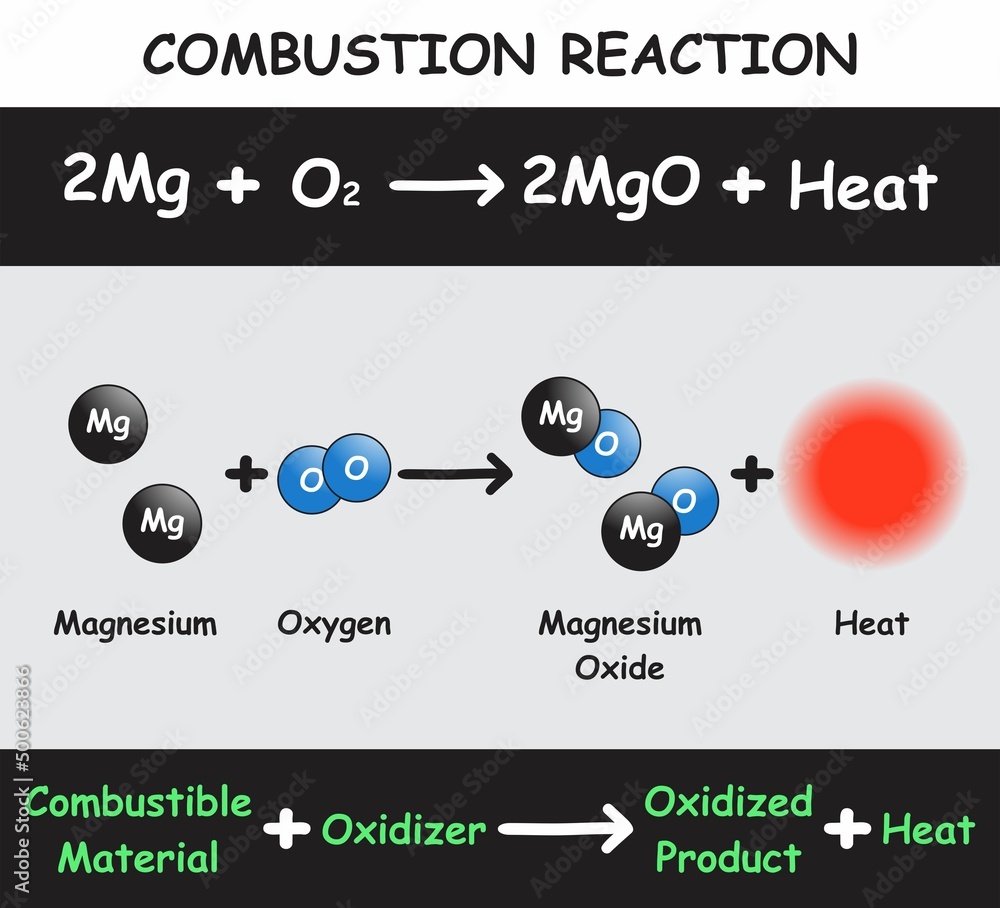
Combustion Reaction Infographic Diagram with example of magnesium

Combustion reaction Royalty Free Vector Image VectorStock
/methanecombustion-58e3e6005f9b58ef7e0daa10.jpg)
An Introduction to Combustion (Burning) Reactions
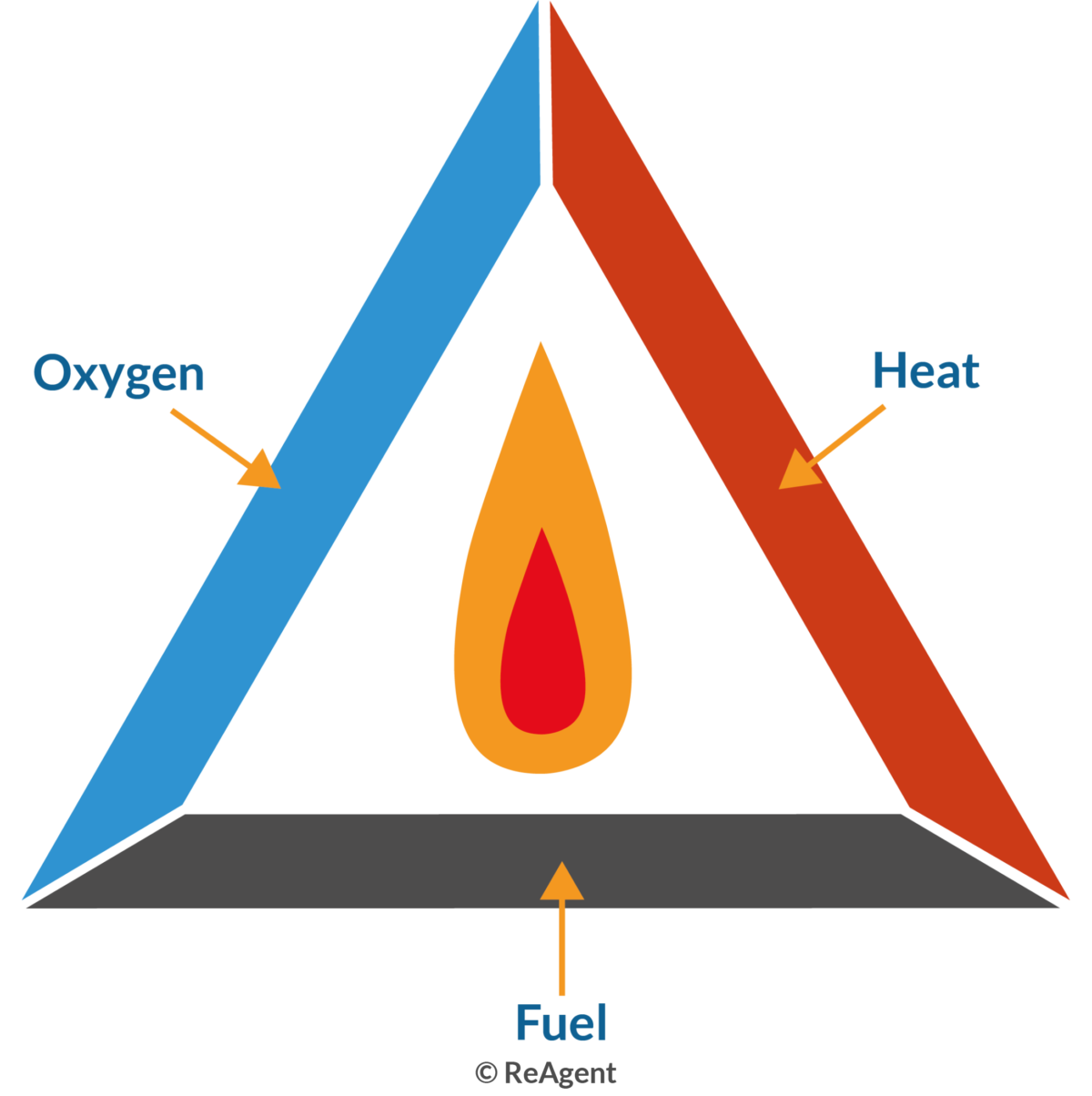
What is Combustion in Chemistry? The Chemistry Blog
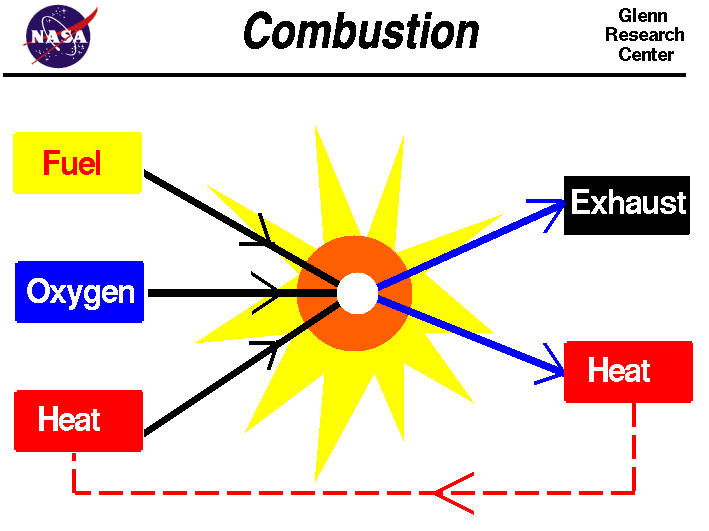
Combustion

Combustion Reactions Introduction, Examples, & Facts What's Insight

Combustion Chemical Reactions, Heat, Oxidation Britannica
Web Butane (C4H10) + Oxygen Æ.
Enthalpy Of Reaction, Formation, And Combustion.
Complete And Balance Each Combustion Equation.
Ch4 (G) + 2O2 (G) → Co2 (G) + 2H2O (L) Step 2:
Related Post: