Clinical Trial Report Template
Clinical Trial Report Template - If none were used, this should be stated. Web evaluation (10 minutes) what is a clinical study report (csr)? Web the clinical study report (csr) is arguably the most important document emerging from a clinical trial. Web the quality assurance and quality control systems implemented to assure the quality of the data should be described in brief. Web novartis staff analyzed this study and authored this report. Learn how to prepare tables, listings, and figures for clinical study report and submit to regulatory agencies, the. This document aims to allow the compilation of a single core clinical study report acceptable to all. “integrated full report of an individual study of any therapeutic, prophylactic or diagnostic agent (referred to herein as. Web protocol templates for clinical trials. Web as such, this csr template is the foundation for an “integrated” full report of any study with a therapeutic, prophylactic, or diagnostic agent (i.e., drug or treatment) conducted in. Web anatomy of a clinical trial: This document aims to allow the compilation of a single core clinical study report acceptable to all. Web clinical study report (csr) is one of many types of regulatory documents that comprise a marketing application for a drug, biologic, or device. If none were used, this should be stated. Nih applicants can use a. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of. Web as such, this csr template is the foundation for an “integrated” full report of any study with a therapeutic, prophylactic, or diagnostic agent (i.e., drug or treatment) conducted in. Web the integrated full report of a study should. Web topics included in the report guide cover reporting checklists, trial report structure, choice of title, writing style, trial registry and reporting consistency, spin or reporting. If none were used, this should be stated. Web r for clinical study reports and submission. A csr is a descriptive account of a. Web evaluation (10 minutes) what is a clinical study report. This document aims to allow the compilation of a single core clinical study report acceptable to all. Web protocol templates for clinical trials. Web protocol templates for clinical trials. Web the quality assurance and quality control systems implemented to assure the quality of the data should be described in brief. “integrated full report of an individual study of any therapeutic,. This guideline can be found. This template aims to facilitate the development of phase. Web the quality assurance and quality control systems implemented to assure the quality of the data should be described in brief. Although this guideline is mainly aimed at efficacy and. Web r for clinical study reports and submission. Web the integrated full report of a study should not be derived by simply joining a separate clinical and statistical report. Web print these checklists, templates, and examples to help gather information needed to report results to clinicaltrials.gov. “integrated full report of an individual study of any therapeutic, prophylactic or diagnostic agent (referred to herein as. The objective of this. Web protocol templates for clinical trials. Case report form, completion guidelines, case report form design, electronic case report form, standard templates. Web the quality assurance and quality control systems implemented to assure the quality of the data should be described in brief. Web protocol templates for clinical trials. Web as such, this csr template is the foundation for an “integrated”. This document aims to allow the compilation of a single core clinical study report acceptable to all. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of. Learn how to prepare tables, listings, and figures for clinical study report and submit to regulatory agencies, the. Web protocol templates for. The signatures of the principal or coordinating investigator, the sponsor’s responsible medical officer, and the report. Web clinical study report (csr) is one of many types of regulatory documents that comprise a marketing application for a drug, biologic, or device. Web topics included in the report guide cover reporting checklists, trial report structure, choice of title, writing style, trial registry. Web protocol templates for clinical trials. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of. Cloud based technologycapture and managefuture of life scienceagile development “integrated full report of an individual study of any therapeutic, prophylactic or diagnostic agent (referred to herein as. This template aims to facilitate the. The purpose, people, and phases of clinical research from 2010 to july 2021, clinicaltrials.gov reports that the number of registered clinical trials. Web clinical study report (csr) is one of many types of regulatory documents that comprise a marketing application for a drug, biologic, or device. Web anatomy of a clinical trial: Web novartis staff analyzed this study and authored this report. Web protocol templates for clinical trials. Web the sap expands the statistical section of the protocol and contains a detailed description of methods to analyze data collected in the study. Web the toolbox contains templates, sample forms, guidelines, regulations and informational materials to assist investigators in the development and conduct of high quality clinical. Web topics included in the report guide cover reporting checklists, trial report structure, choice of title, writing style, trial registry and reporting consistency, spin or reporting. This guideline can be found. Cloud based technologycapture and managefuture of life scienceagile development A csr is a descriptive account of a. Forms, tools, & templates description category (ies) keyword (s) 02.04.02 investigator's brochure. Web as such, this csr template is the foundation for an “integrated” full report of any study with a therapeutic, prophylactic, or diagnostic agent (i.e., drug or treatment) conducted in. The objective of this guideline is to facilitate the compilation of a single core clinical study report acceptable to all regulatory authorities. “integrated full report of an individual study of any therapeutic, prophylactic or diagnostic agent (referred to herein as. This document aims to allow the compilation of a single core clinical study report acceptable to all.
Clinical Trial Report Template

Case Report Form Template Clinical Trials
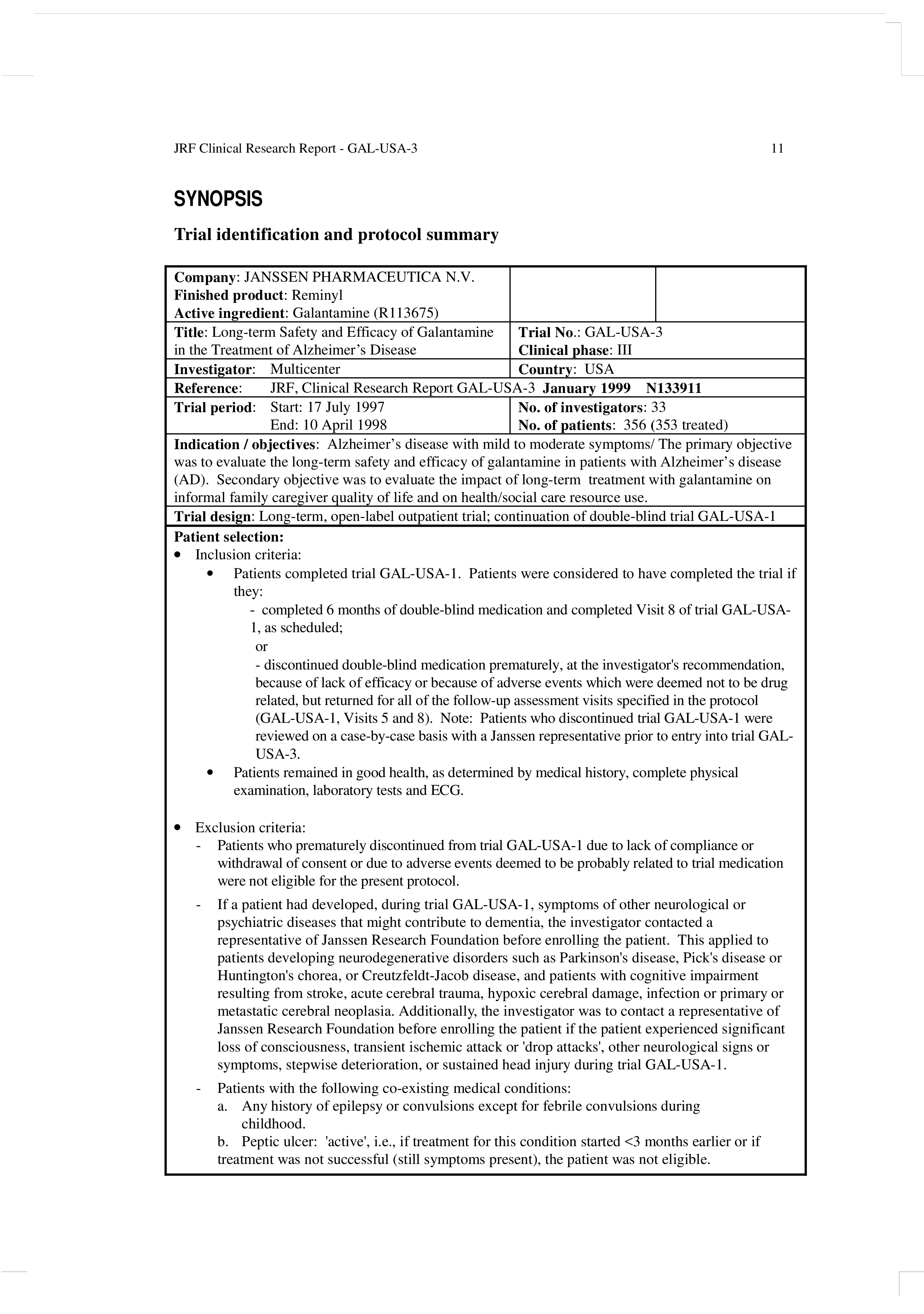
Clinical Research Report Synopsis Templates at

Clinical Trial Report Template (3) PROFESSIONAL TEMPLATES
Clinical Study Report Template PDF Sample Stableshvf

Free Clinical Trial Templates Smartsheet
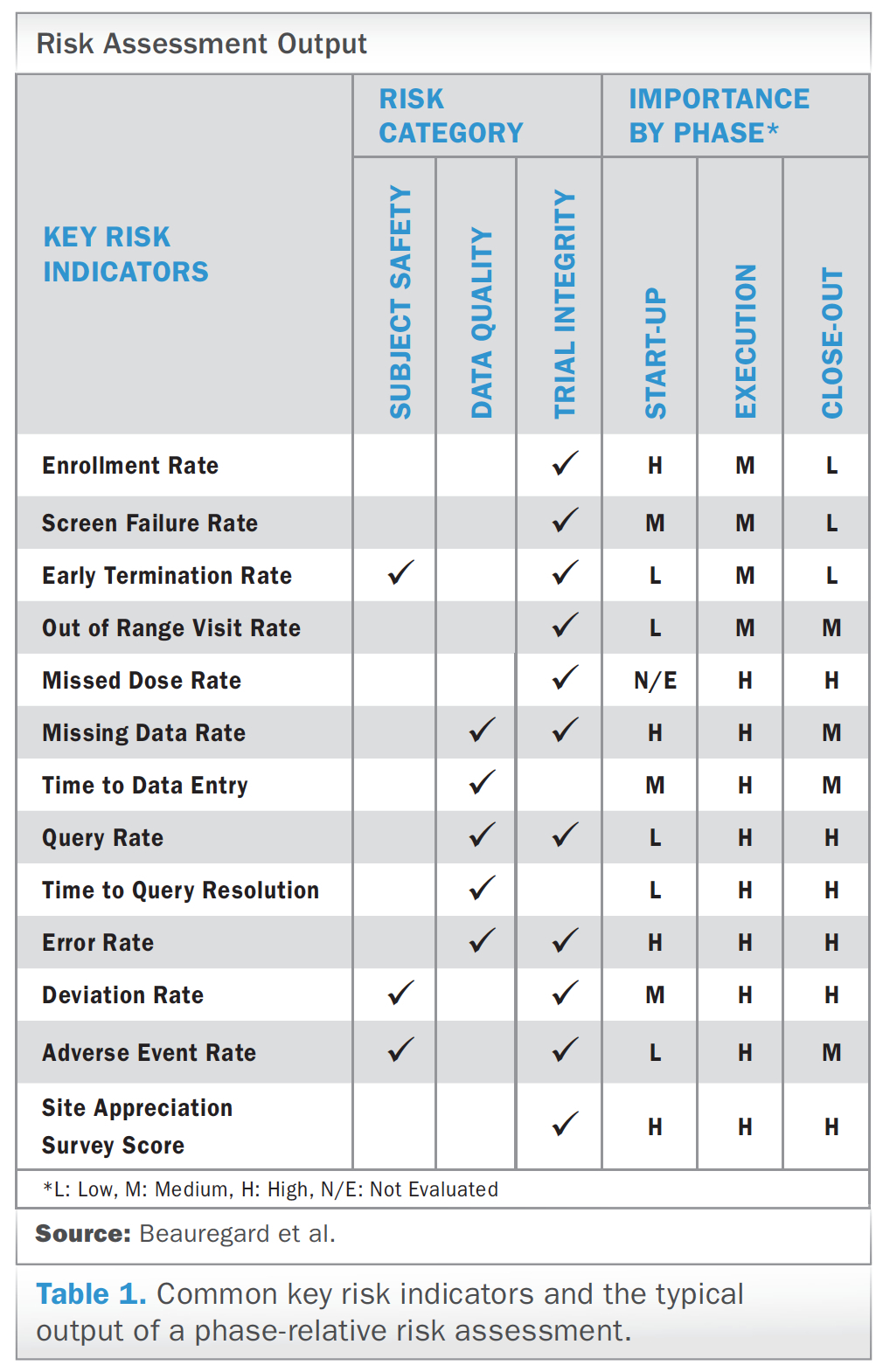
Monitoring Report Template Clinical Trials

Clinical Trial Report Template TEMPLATES EXAMPLE TEMPLATES EXAMPLE

Clinical Trial Report Template 3 Templates Example Templates
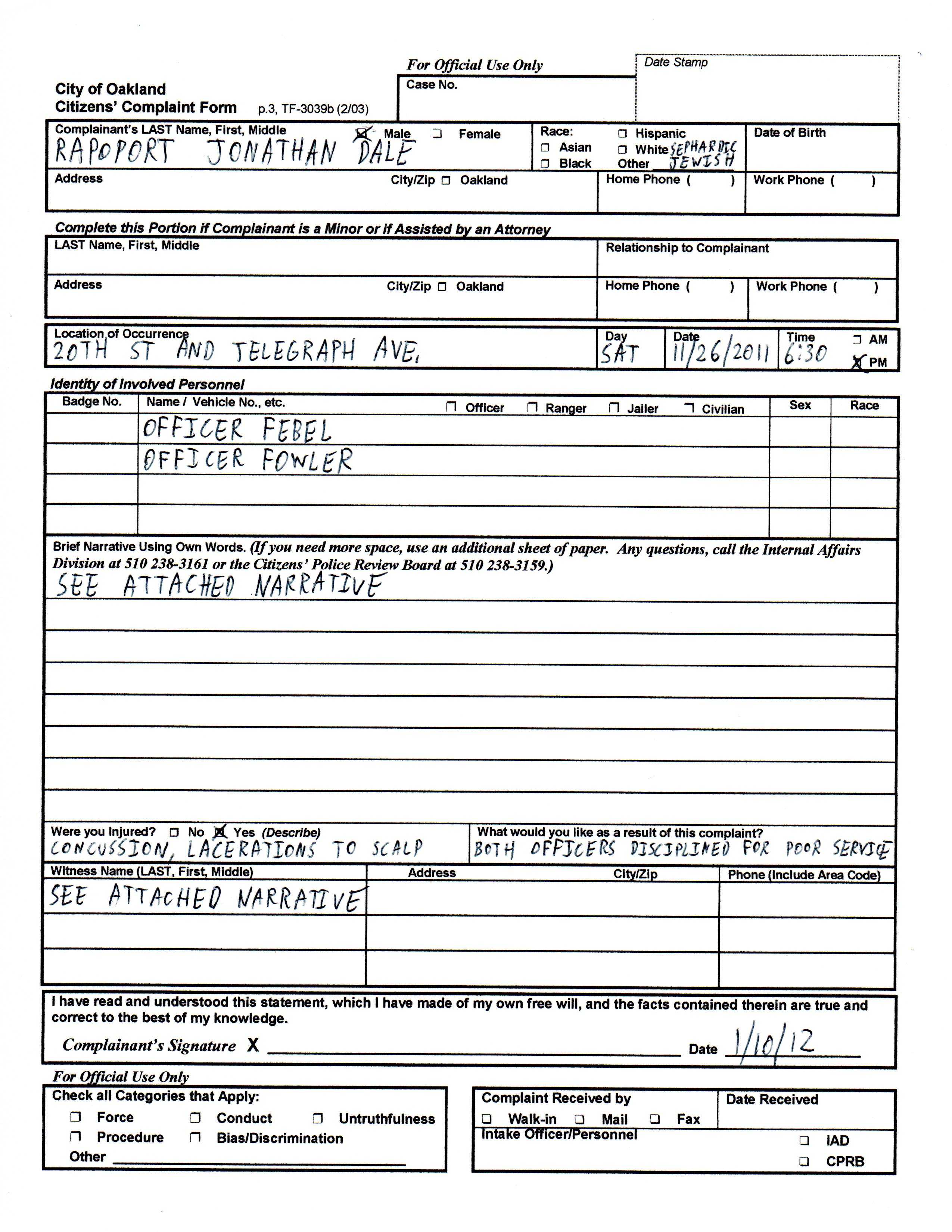
Case Eport Form Electronic Qolty Format Ppt In Clinical With Trial
Please Note That This Page Has Been Updated For 2015 Following A Quality Check And Review Of The Templates, And.
Web Protocol Templates For Clinical Trials.
If None Were Used, This Should Be Stated.
Web Evaluation (10 Minutes) What Is A Clinical Study Report (Csr)?
Related Post: