Clinical Study Protocol Template
Clinical Study Protocol Template - Clinical electronic structured harmonised protocol december 2022. The simple innovation is to include all. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national institutes of health (nih) that are being conducted under a food. Trials is experimenting with a new way of structuring study protocols for randomised trials. Web the ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical protocol organization with standardized content with. They follow the format of typical nih and industry multicenter protocols. Web using protocol templates, you can start thinking through what you need to meet compliance standards with the food and drug administration (fda) and clinical. Web original study protocol and the final protocol with a summary of changes. Web this protocol template is a tool to facilitate the development of a research study protocol specifically designed for the investigator initiated studies. Web sample protocol templates and resources: Clinical trial protocol template version 3.4 (may 2017). Web structured study protocol template | trials. They follow the format of typical nih and industry multicenter protocols. Web this protocol template is a tool to facilitate the development of a research study protocol specifically designed for the investigator initiated studies. Web suggested templates for phase 1 and 2 clinical trials. Web the ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical protocol organization with standardized content with. The simple innovation is to include all. Web 31 • because the scope of this protocol template is focused on interventional clinical trials, 32 the term clinical trials is used rather than clinical studies when referring to 33. Web this protocol. Web the irb provides several protocol templates on this page. Web this protocol template is a tool to facilitate the development of a research study protocol specifically designed for the investigator initiated studies. Clinical trial protocol clik066b2204 / nct03152552. Web sample protocol templates and resources: Web clinical trial protocol cain457ade11c / nct03765788. It contains sample text to assist. Clinical trial protocol clik066b2204 / nct03152552. Web 31 • because the scope of this protocol template is focused on interventional clinical trials, 32 the term clinical trials is used rather than clinical studies when referring to 33. Generic protocol documents and instructions for ctep studies. Web original study protocol and the final protocol with. Web 31 • because the scope of this protocol template is focused on interventional clinical trials, 32 the term clinical trials is used rather than clinical studies when referring to 33. Original reporting and analysis plan (statistical analysis plan), which is the final statistical Web clinical trial protocol cain457ade11c / nct03765788. Clinical trial protocol clik066b2204 / nct03152552. Web original study. Download the draft guidance document. Web structured study protocol template | trials. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national institutes of health (nih) that are being conducted under a food. Web clinical trial protocol template version 3.2 ( july 2016) novartis confidential page 2 clinical trial. Web 31 • because the scope of this protocol template is focused on interventional clinical trials, 32 the term clinical trials is used rather than clinical studies when referring to 33. Trials is experimenting with a new way of structuring study protocols for randomised trials. Web clinical trial protocol template version 3.2 ( july 2016) novartis confidential page 2 clinical. Web 31 • because the scope of this protocol template is focused on interventional clinical trials, 32 the term clinical trials is used rather than clinical studies when referring to 33. Clinical trial protocol clik066b2204 / nct03152552. Web original study protocol and the final protocol with a summary of changes. The simple innovation is to include all. Web the irb. The simple innovation is to include all. Clinical trial protocol template version 3.4 (may 2017). Download the draft guidance document. Generic protocol documents and instructions for ctep studies. Web suggested templates for phase 1 and 2 clinical trials. It contains sample text to assist. Web the ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical protocol organization with standardized content with. Web this protocol template is a tool to facilitate the development of a research study protocol specifically designed for the investigator initiated studies. Trials is experimenting with a new way of structuring study protocols for. Web the ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical protocol organization with standardized content with. Clinical electronic structured harmonised protocol december 2022. Web clinical trial protocol template version 3.2 ( july 2016) novartis confidential page 2 clinical trial protocol (version 00) protocol no. Clinical trial protocol clik066b2204 / nct03152552. Download the draft guidance document. Web structured study protocol template | trials. Web 31 • because the scope of this protocol template is focused on interventional clinical trials, 32 the term clinical trials is used rather than clinical studies when referring to 33. Web this protocol template is a tool to facilitate the development of a research study protocol specifically designed for the investigator initiated studies. Trials is experimenting with a new way of structuring study protocols for randomised trials. Web using protocol templates, you can start thinking through what you need to meet compliance standards with the food and drug administration (fda) and clinical. It contains sample text to assist. They follow the format of typical nih and industry multicenter protocols. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national institutes of health (nih) that are being conducted under a food. Web suggested templates for phase 1 and 2 clinical trials. Web the irb provides several protocol templates on this page. Web clinical trial protocol cain457ade11c / nct03765788.
Clinical Interventional Study Protocol Template Doc Template pdfFiller

Clinical Study Protocol (CSP) Template Clinical Study Templates
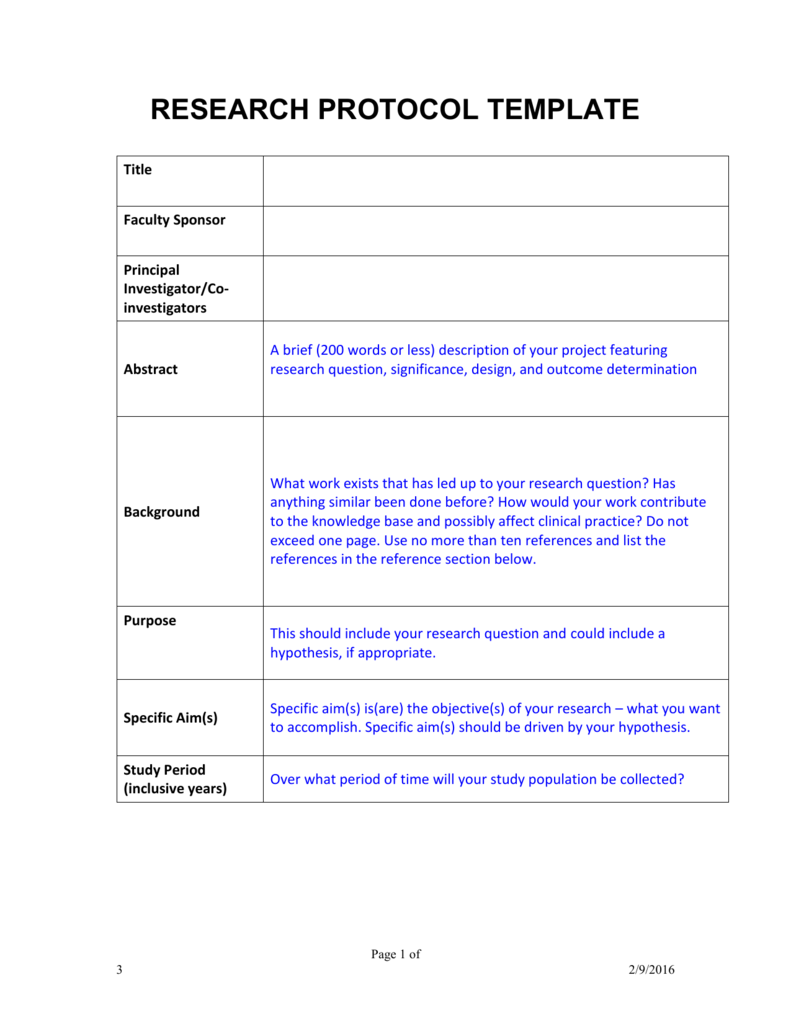
research protocol template

Clinical Trial Protocol Template Word

Study Protocol Template

TEMPLATE CLINICAL STUDY PROTOCOL

Clinical Study Protocol Template
Phylotocol template. Based on the NIH clinical trial protocol, the
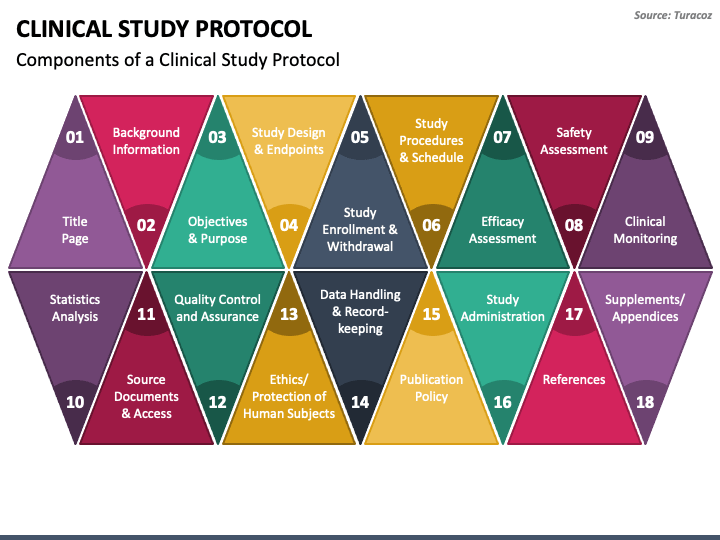
Clinical Study Protocol PowerPoint Template PPT Slides

WA Health Research Protocol Template for Clinical Trials
Web Original Study Protocol And The Final Protocol With A Summary Of Changes.
The Simple Innovation Is To Include All.
Original Reporting And Analysis Plan (Statistical Analysis Plan), Which Is The Final Statistical
Clinical Trial Protocol Template Version 3.4 (May 2017).
Related Post: