Clinical Evaluation Report Template
Clinical Evaluation Report Template - Web medical device clinical evaluation report (cer) rough template july 2020. Clinical evaluation report is a document that has all necessary elements for conducting and reporting the. It is primarily aimed at clinical evaluation reviewers, particularly. Web download the template for clinical evaluation assessment report, a document required by eu medical device regulation. Web a clinical evaluation report (cer) is a comprehensive document that summarizes the results of the clinical evaluation process for a medical device. By ramya sriram on may 14, 2020 medical devices. Web download a pdf template for clinical evaluation report of medical devices based on eu regulation. Open talent platform kolabtree has announced the release of an expert. Web clinical evaluation report template: Web learn how to conduct a clinical evaluation of a medical device using scientifically sound methods and documentation. Web clinical evaluation report template: Open talent platform kolabtree has announced the release of an expert. By kolabtree 13 jan 2022. Web a clinical evaluation report (cer) is a comprehensive document that summarizes the results of the clinical evaluation process for a medical device. Find out what makes cer challenging, how to use a template effectively,. Web iso 13485 / mdr document template: Web download the template for clinical evaluation assessment report, a document required by eu medical device regulation. Web medical device clinical evaluation report (cer) rough template july 2020. Web the end result of this process is the production of a clinical evaluation report (cer). A free resource for medical device manufacturers. Girish hirpara, regulatory consultant on kolabtree, provides a clinical evaluation report sample for medical. According to these guidelines, you arrive at this cer by the following process: A free resource for medical device manufacturers. By kolabtree 13 jan 2022. Web clinical evaluation report template: 5+ clinical evaluation report templates. According to the regulation (eu) 2017/745, article 61 and annex xiv, the evaluation of the clinical performance and safety as well as the clinical benefit must be based on clinical data and is required for all medical device classes. Girish hirpara, regulatory consultant on kolabtree, provides a clinical evaluation report sample for medical. Web a. Web download a pdf template for clinical evaluation report of medical devices based on eu regulation. This document provides a template and guidance for the. Web clinical evaluation report template: The template is arranged into numbered. Web medical device clinical evaluation report (cer) rough template july 2020. A free resource for medical device manufacturers. By ramya sriram on may 14, 2020 medical devices. The template is arranged into numbered. By kolabtree 13 jan 2022. According to these guidelines, you arrive at this cer by the following process: The template covers product information, clinical benefits, clinical claims,. Web medical device clinical evaluation report (cer) rough template july 2020. Web download a pdf template for clinical evaluation report of medical devices based on eu regulation. This document provides a template and guidance for the. This guidance is endorsed by the. The template is arranged into numbered. This guidance is endorsed by the. By ramya sriram on may 14, 2020 medical devices. It is primarily aimed at clinical evaluation reviewers, particularly. Open talent platform kolabtree has. Open talent platform kolabtree has announced the release of an expert. Clinical evaluation report is a document that has all necessary elements for conducting and reporting the. Web the end result of this process is the production of a clinical evaluation report (cer). According to the regulation (eu) 2017/745, article 61 and annex xiv, the evaluation of the clinical performance. This document provides a template and guidance for the. A free resource for medical device manufacturers. 5+ clinical evaluation report templates. The template covers product information, clinical benefits, clinical claims,. Web download the template for clinical evaluation assessment report, a document required by eu medical device regulation. Web download a pdf template for clinical evaluation report of medical devices based on eu regulation. According to these guidelines, you arrive at this cer by the following process: It is primarily aimed at clinical evaluation reviewers, particularly. According to the regulation (eu) 2017/745, article 61 and annex xiv, the evaluation of the clinical performance and safety as well as the clinical benefit must be based on clinical data and is required for all medical device classes. Web a document endorsed by the mdcg that provides a standardised method for notified bodies to document their assessment of manufacturers' clinical evaluation and related. Girish hirpara, regulatory consultant on kolabtree, provides a clinical evaluation report sample for medical. Clinical evaluation report is a document that has all necessary elements for conducting and reporting the. Open talent platform kolabtree has. The template covers product information, clinical benefits, clinical claims,. By ramya sriram on may 14, 2020 medical devices. Web how to use this template. Web clinical evaluation report template: 5+ clinical evaluation report templates. This guidance is endorsed by the. This document provides a template and guidance for the. To read the file of this research, you can.
25+ Medical Report Samples Word, PDF Free & Premium Templates

Free Printable Nursing Assessment Forms Printable Forms Free Online

FREE 15+ Sample Evaluation Reports in PDF MS Word Apple Pages
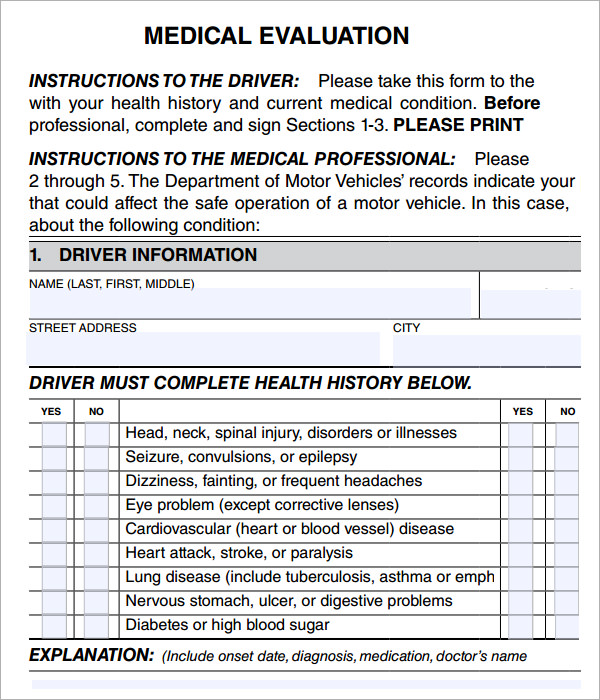
6 Sample Medical Evaluation Templates to Download Sample Templates

FREE 7+ Medical Evaluation Forms in PDF MS Word

Clinical Evaluation Report Template Glendale Community regarding
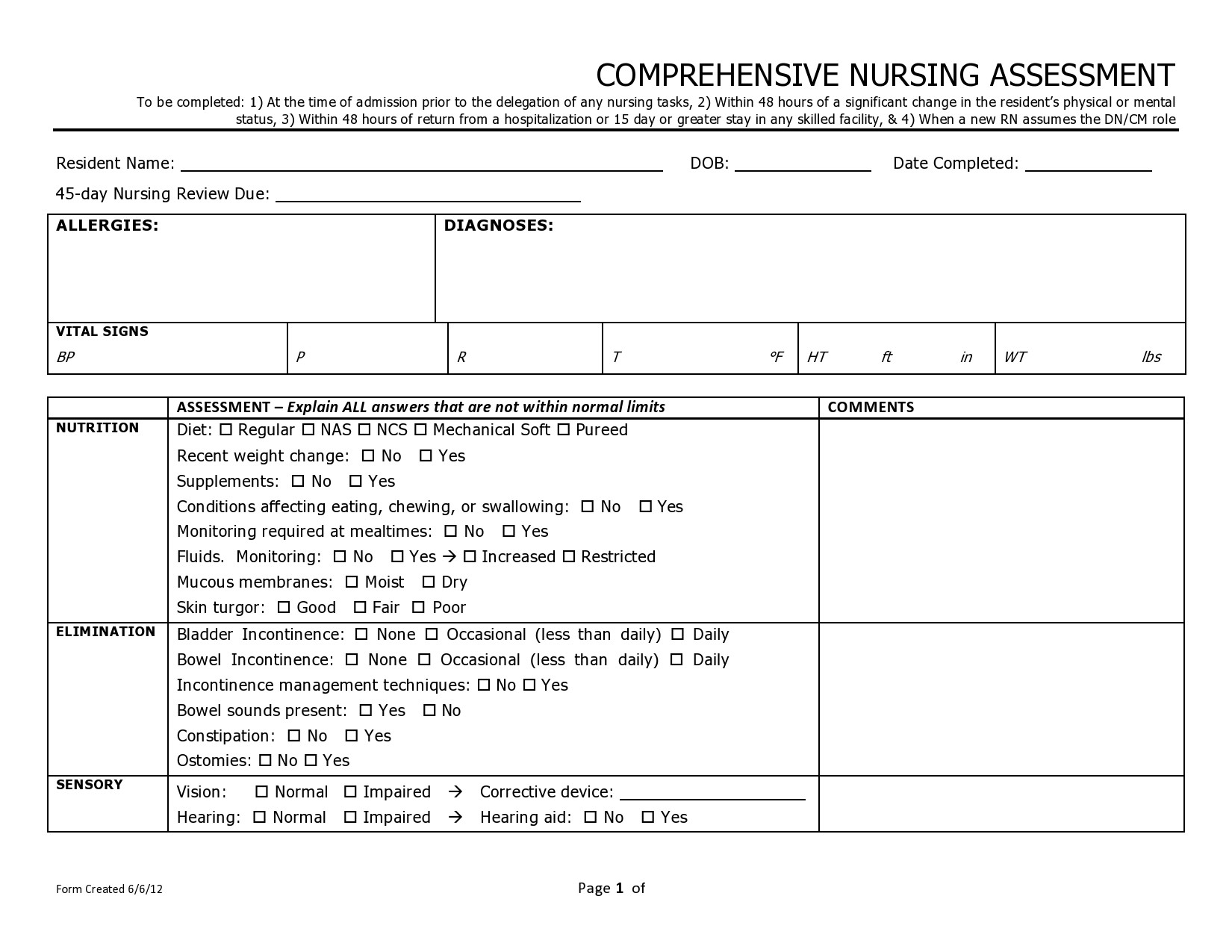
39 Printable Nursing Assessment Forms (+Examples)

Clinical Evaluation Plan/Report Fill and Sign Printable Template

Clinical Evaluation Report Template

Clinical Evaluation Report Template
Find Out What Makes Cer Challenging, How To Use A Template Effectively,.
The Template Is Arranged Into Numbered.
Web Iso 13485 / Mdr Document Template:
Web Download The Template For Clinical Evaluation Assessment Report, A Document Required By Eu Medical Device Regulation.
Related Post: