Clinical Development Plan Template
Clinical Development Plan Template - It outlines the clinical program design, including development,. A clinical development plan (cdp) is key to bringing an investigational medicinal product (imp) to. It should consider current knowledge about the condition being treated, the target patient. Web this section is intended to place the clinical development plan for the investigational new drug into perspective and to help fda anticipate the needs of the. The medical device regulation (mdr) mentions the term “clinical development plan” (cdp) only twice,. Web the clinical development plan is the blueprint of the entire clinical research strategy of a drug which defines the critical path for the clinical program including. Web easily editable, printable, downloadable. Powerpoint presentations can and should be used by all members of the clinical development team. Web the clinical development plan is the blueprint of the entire clinical research strategy of a drug, which defines the critical path for the clinical program, including. Cleartrial provides four edit modes that govern the. Web the development and evolution of a clinical development plan (cdp) is a critical document and process for cost effective and efficient drug development programs. Web the clinical development plan is the blueprint of the entire clinical research strategy of a drug which defines the critical path for the clinical program including. Web this article is intended to assist medical. Our clinical development plan template lets you create a developmental plan specifically for clinical purposes. Web clinical development plan (cdp) design. It should consider current knowledge about the condition being treated, the target patient. Web key components of the clinical development plan (see below) prospective patient package insert. Web a strategic, comprehensive clinical development plan (cdp) can help sponsors optimize. Conduct literature review on target indication. Web the development and evolution of a clinical development plan (cdp) is a critical document and process for cost effective and efficient drug development programs. Web this section is intended to place the clinical development plan for the investigational new drug into perspective and to help fda anticipate the needs of the. Powerpoint presentations. Web the clinical development plan is the blueprint of the entire clinical research strategy of a drug which defines the critical path for the clinical program including. Web the clinical development plan is the blueprint of the entire clinical research strategy of a drug, which defines the critical path for the clinical program, including. The cdp is the blueprint of. Web clinical development plan (cdp) design. Clinical development project management plan. Web the clinical development plan is the blueprint of the entire clinical research strategy of a drug, which defines the critical path for the clinical program, including. Web this white paper provides tips and best practices on developing and executing a clinical strategy: The clinical evaluation plan defines methods. Web what is a clinical development strategic plan? Conduct literature review on target indication. Web clinical development plan (cdp) design. Clinical development project management plan. Powerpoint presentations can and should be used by all members of the clinical development team. Web this section is intended to place the clinical development plan for the investigational new drug into perspective and to help fda anticipate the needs of the. Conduct literature review on target indication. The medical device regulation (mdr) mentions the term “clinical development plan” (cdp) only twice,. Web the clinical development plan is the blueprint of the entire clinical research. It should consider current knowledge about the condition being treated, the target patient. The medical device regulation (mdr) mentions the term “clinical development plan” (cdp) only twice,. Web clinical development plan (cdp) design. The cdp is the blueprint of a drug’s entire clinical research strategy. Clinical development project management plan. A clinical development plan (cdp) is key to bringing an investigational medicinal product (imp) to. Cleartrial provides four edit modes that govern the. The cdp is the blueprint of a drug’s entire clinical research strategy. Web a strategic, comprehensive clinical development plan (cdp) can help sponsors optimize efficiency, control costs, plan timelines, and maximize the probability. Web this white paper. Web tailor a clinical development plan to the specific needs of the treatment being developed. Web this section is intended to place the clinical development plan for the investigational new drug into perspective and to help fda anticipate the needs of the. The clinical evaluation plan defines methods for creating and updating the clinical evaluation report. Web this article is. Clinical development project management plan. Web what is a clinical development strategic plan? Web this section is intended to place the clinical development plan for the investigational new drug into perspective and to help fda anticipate the needs of the. Web a strategic, comprehensive clinical development plan (cdp) can help sponsors optimize efficiency, control costs, plan timelines, and maximize the probability. Web clinical development plan • generate cdp scenarios • timelines and key milestones • endpoint strategy • regulatory strategy • statistical modeling • study design concept. Web the clinical development plan. Web this article is intended to assist medical device manufacturers and medical writers to leverage the cdp as a tool to showcase their clinical evaluation strategy and plan. Web the clinical development plan is the blueprint of the entire clinical research strategy of a drug which defines the critical path for the clinical program including. Web key components of the clinical development plan (see below) prospective patient package insert. Web clinical development plan templates to deliver with precision. Web the clinical development plan is the blueprint of the entire clinical research strategy of a drug, which defines the critical path for the clinical program, including. It outlines the clinical program design, including development,. The medical device regulation (mdr) mentions the term “clinical development plan” (cdp) only twice,. Powerpoint presentations can and should be used by all members of the clinical development team. The clinical evaluation plan defines methods for creating and updating the clinical evaluation report. Web tailor a clinical development plan to the specific needs of the treatment being developed.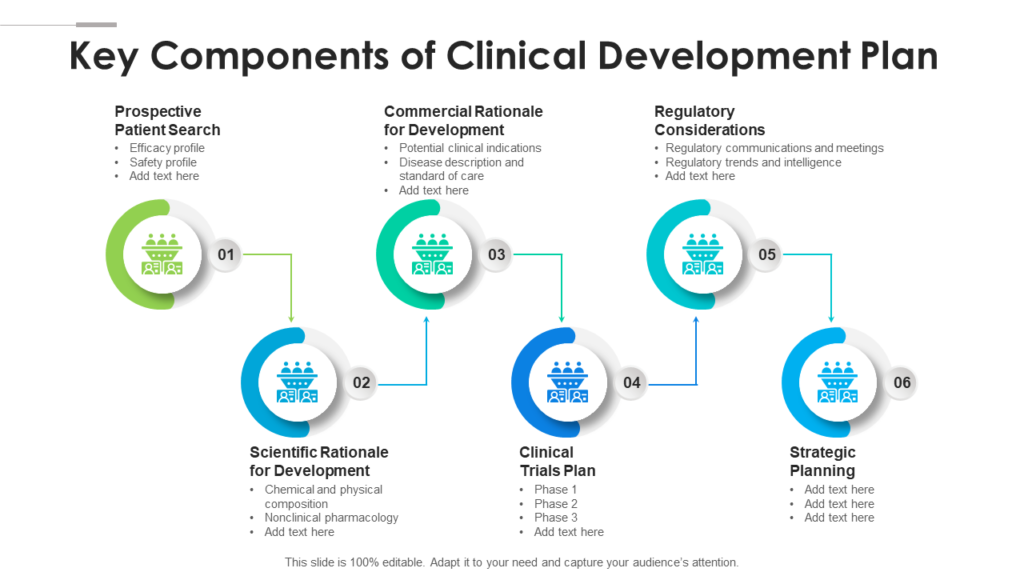
Top 20 PowerPoint Templates to Create a Clinical Development Plan

Clinical Development Plan Template Google Docs, Word, Apple Pages

Clinical Trials Strategy The Clinical Development Plan

Clinical Development Plan Template in Google Docs, Word, Apple Pages
Top 20 PowerPoint Templates to Create a Clinical Development Plan
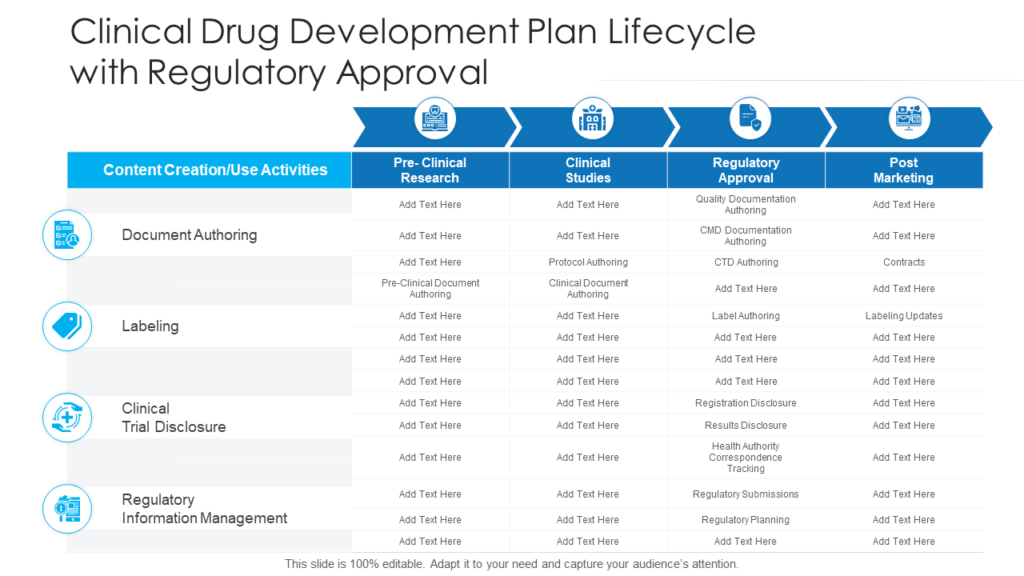
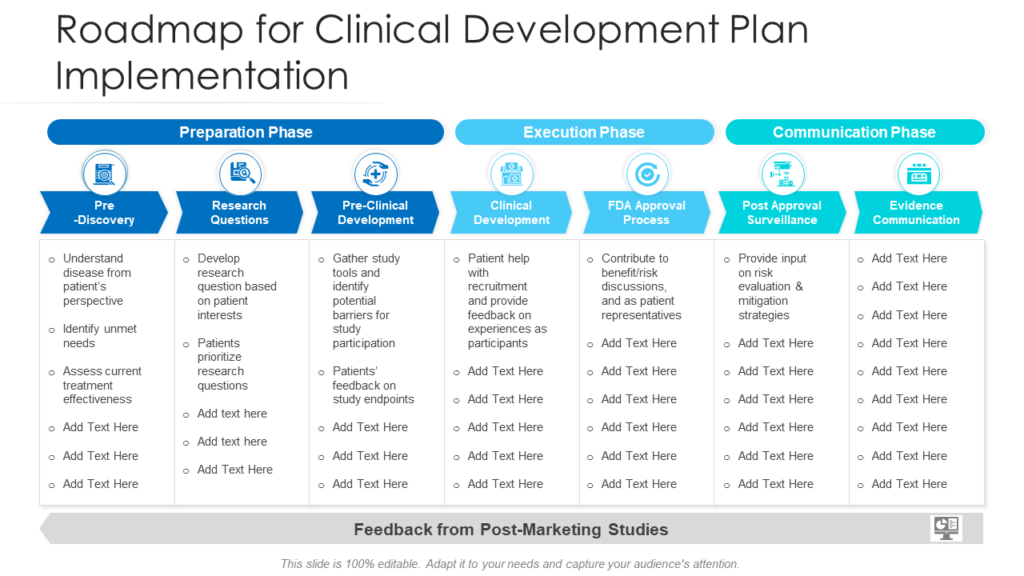
Top 20 PowerPoint Templates to Create a Clinical Development Plan
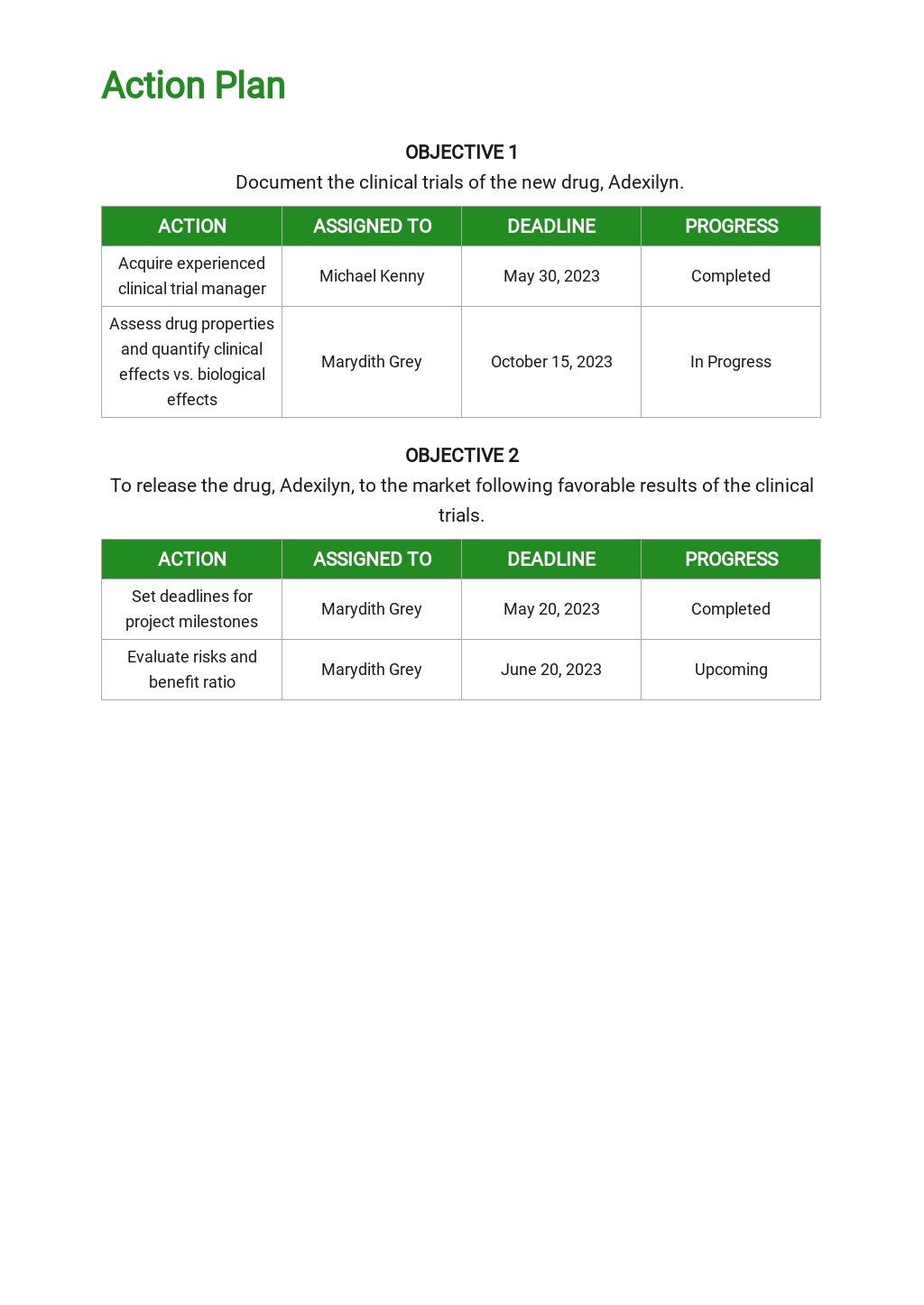
Clinical Development Plan Template in Google Docs, Word, Apple Pages

Clinical Development Plan Template Download in Word, Google Docs

Clinical Development Plan Template Venngage
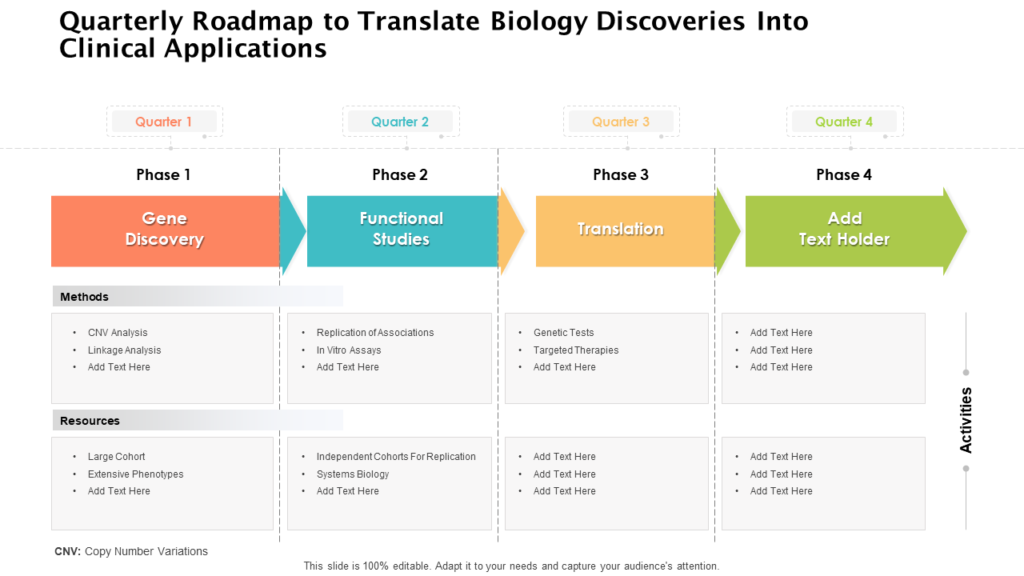
Top 20 PowerPoint Templates to Create a Clinical Development Plan
Our Clinical Development Plan Template Lets You Create A Developmental Plan Specifically For Clinical Purposes.
A Good Way To Make Your Employees Happy At.
Web This White Paper Provides Tips And Best Practices On Developing And Executing A Clinical Strategy:
Web Clinical Development Plan (Cdp) Design.
Related Post: