Capa Template
Capa Template - Web include in your capa reports. If you are a user of formwork, our eqms software, choose “qms” on the top menu and “openregulatory. Web get started with this customizable capa form template. Web learn how to use corrective and preventive action (capa) to improve your organization's quality and performance. [date that the capa is written] to:. Corrective and preventive actions (capa) inspectional objectives. In your plan, include information that is: Download free capa templates for 5 whys,. Definition, example & template | pm study circle. Identify the actions you or others will take to address the root cause, the. Web the iu hrpp quality improvement office (qio) has developed a capa plan template to assist study teams with this activity. In your plan, include information that is: Web get started with this customizable capa form template. Identify the actions you or others will take to address the root cause, the. Corrective and preventive actions (capa) are. If you are a user of formwork, our eqms software, choose “qms” on the top menu and “openregulatory. The following is a template for the content and format of a capa to the study investigators. Web get started with this customizable capa form template. Verify that capa system procedure (s) that address the requirements of the quality. Web corrective and. Corrective and preventive actions (capa) form a key approach in quality management systems. Web corrective and preventive action (capa): Web free lean six sigma templates. Web this template provides a detailed guide on how to implement and track capas according to iso 13485:2016. Verify that capa system procedure (s) that address the requirements of the quality. Corrective actions and preventive actions (capa’s) can also be. Corrective and preventive actions (capa) inspectional objectives. Web download corrective and preventative action plan form template_2019.11.13. Web the iu hrpp quality improvement office (qio) has developed a capa plan template to assist study teams with this activity. Web corrective action preventive action (capa) is the result of a us fda requirement,. In your plan, include information that is: Web this template provides a detailed guide on how to implement and track capas according to iso 13485:2016. Web corrective and preventive action (capa): Web get started with this customizable capa form template. Definition, example & template | pm study circle. Web the iu hrpp quality improvement office (qio) has developed a capa plan template to assist study teams with this activity. Web capa plans must be thorough and well documented. It covers the input, output, process steps, participants, and verification. This template is designed to. Web corrective and preventive action (capa): The following is a template for the content and format of a capa to the study investigators. The capa requirement applies to manufacturers of medical devices and. Capa reports are typically initiated in response to. Corrective action and preventive action (capa) plan template. [date that the capa is written] to:. Web this is a free template, provided by openregulatory. This template is designed to. Web the iu hrpp quality improvement office (qio) has developed a capa plan template to assist study teams with this activity. If you are a user of formwork, our eqms software, choose “qms” on the top menu and “openregulatory. Web this template provides a detailed guide. Web include in your capa reports. Corrective and preventive actions (capa) inspectional objectives. It covers the input, output, process steps, participants, and verification. Web this is a free template, provided by openregulatory. Web corrective and preventive action (capa): The capa requirement applies to manufacturers of medical devices and. Identify the actions you or others will take to address the root cause, the. Web include in your capa reports. If you are a user of formwork, our eqms software, choose “qms” on the top menu and “openregulatory. Corrective and preventive actions (capa) inspectional objectives. Web free lean six sigma templates. In your plan, include information that is: If you’ve ever had a workplace incident, you should know how to create a capa. Corrective and preventive actions (capa) form a key approach in quality management systems. It covers the input, output, process steps, participants, and verification. Web capa plans must be thorough and well documented. The capa requirement applies to manufacturers of medical devices and. [date that the capa is written] to:. Capa reports are typically initiated in response to. Download free capa templates for 5 whys,. Definition, example & template | pm study circle. Web the iu hrpp quality improvement office (qio) has developed a capa plan template to assist study teams with this activity. The following is a template for the content and format of a capa to the study investigators. Corrective actions and preventive actions (capa’s) can also be. If you are a user of formwork, our eqms software, choose “qms” on the top menu and “openregulatory. This template is designed to.
CAPA form Corrective action and preventive action

CAPA Beginner’s Guide To Corrective Action Preventive Action
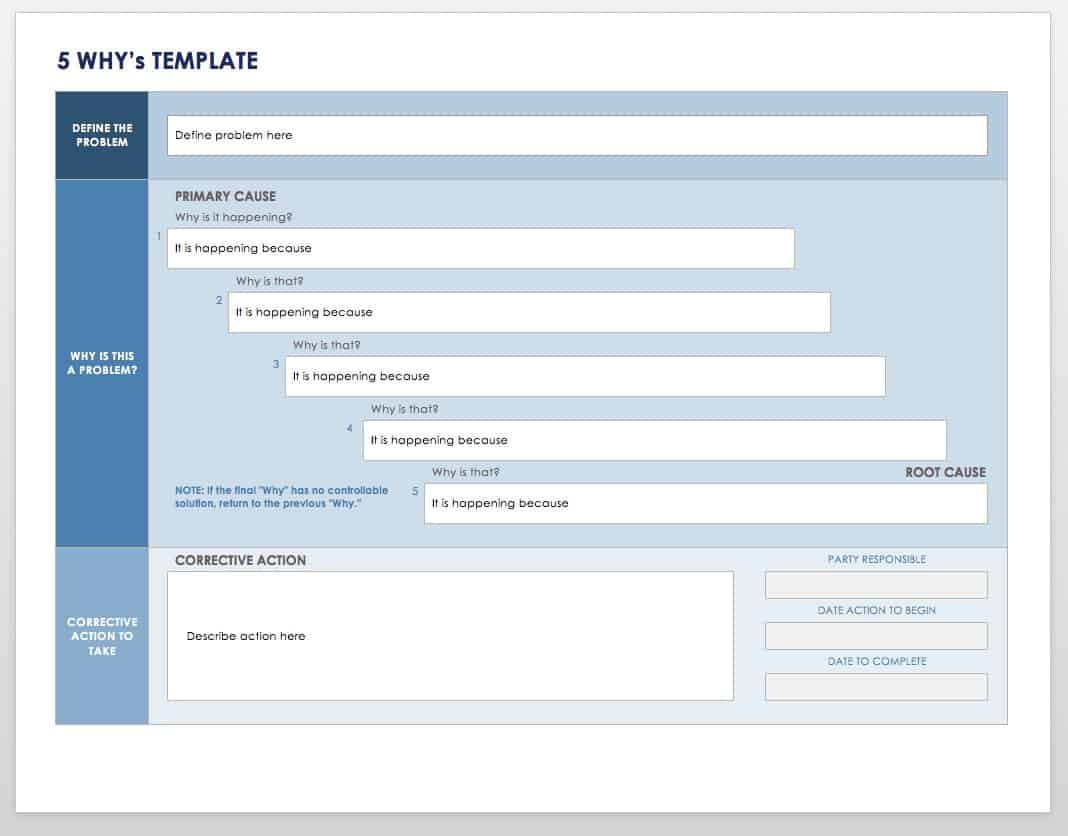
The Beginner’s Guide to CAPA Smartsheet
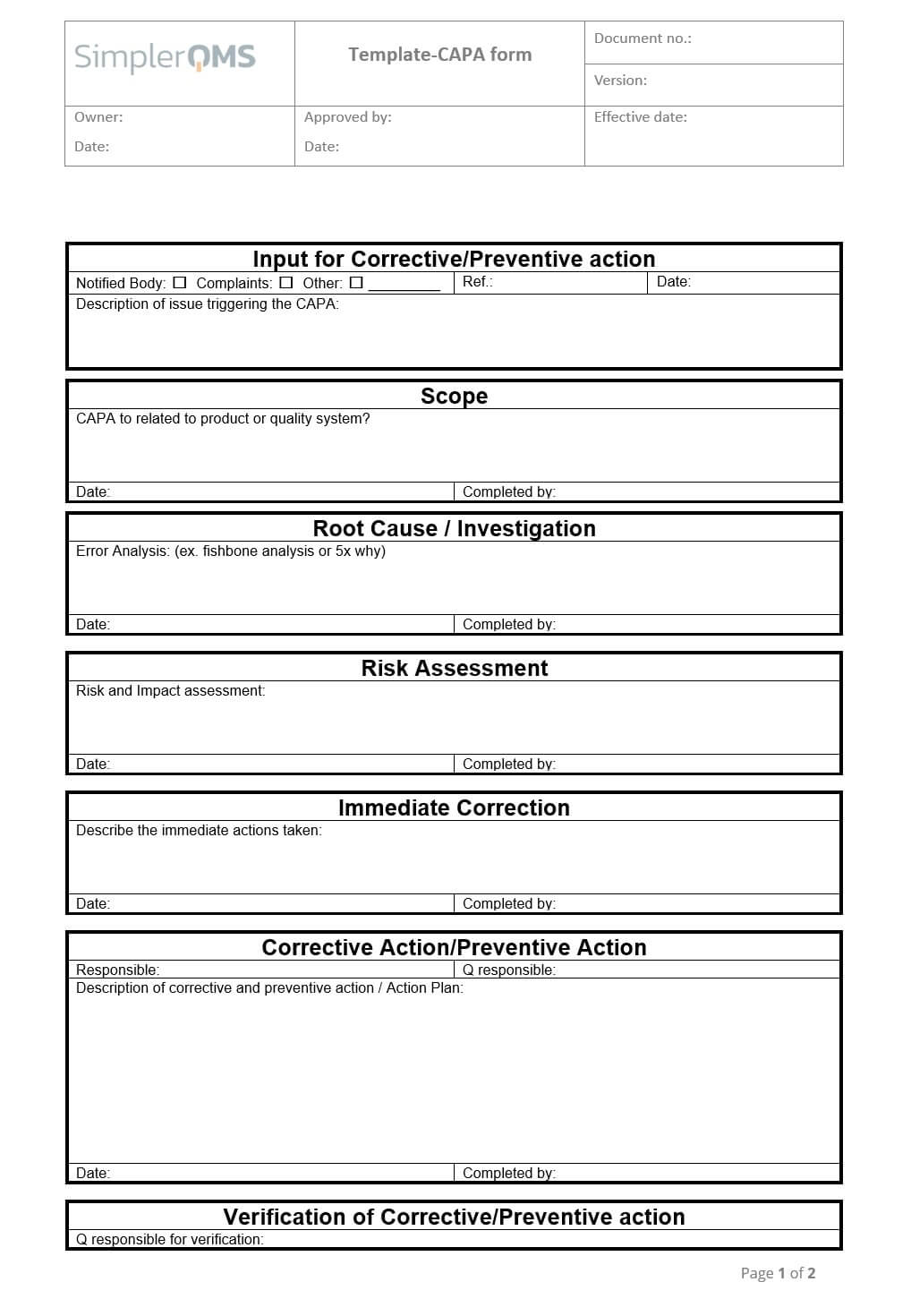
Corrective and Preventive Action (CAPA) Form Template SimplerQMS
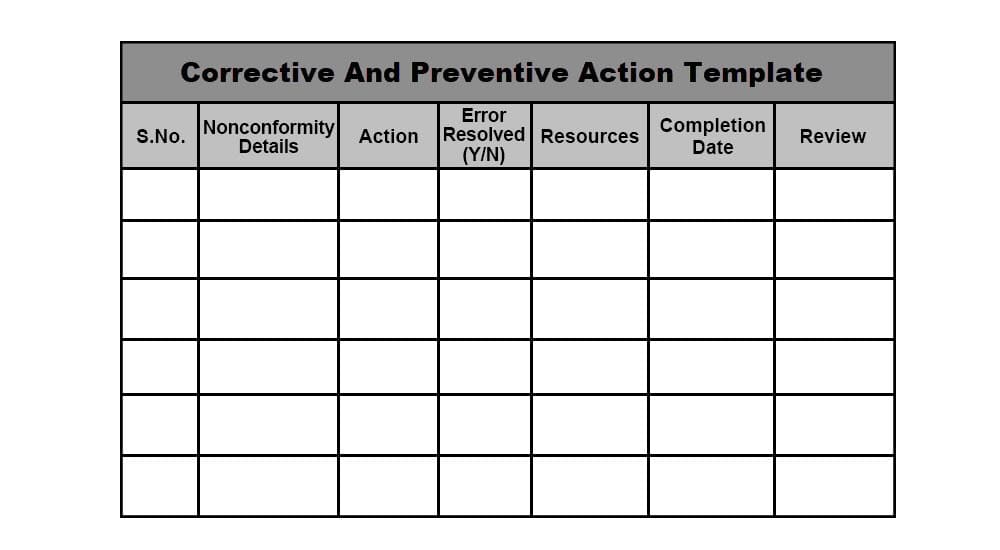
Corrective and Preventive Action (CAPA) Definition, Example & Template
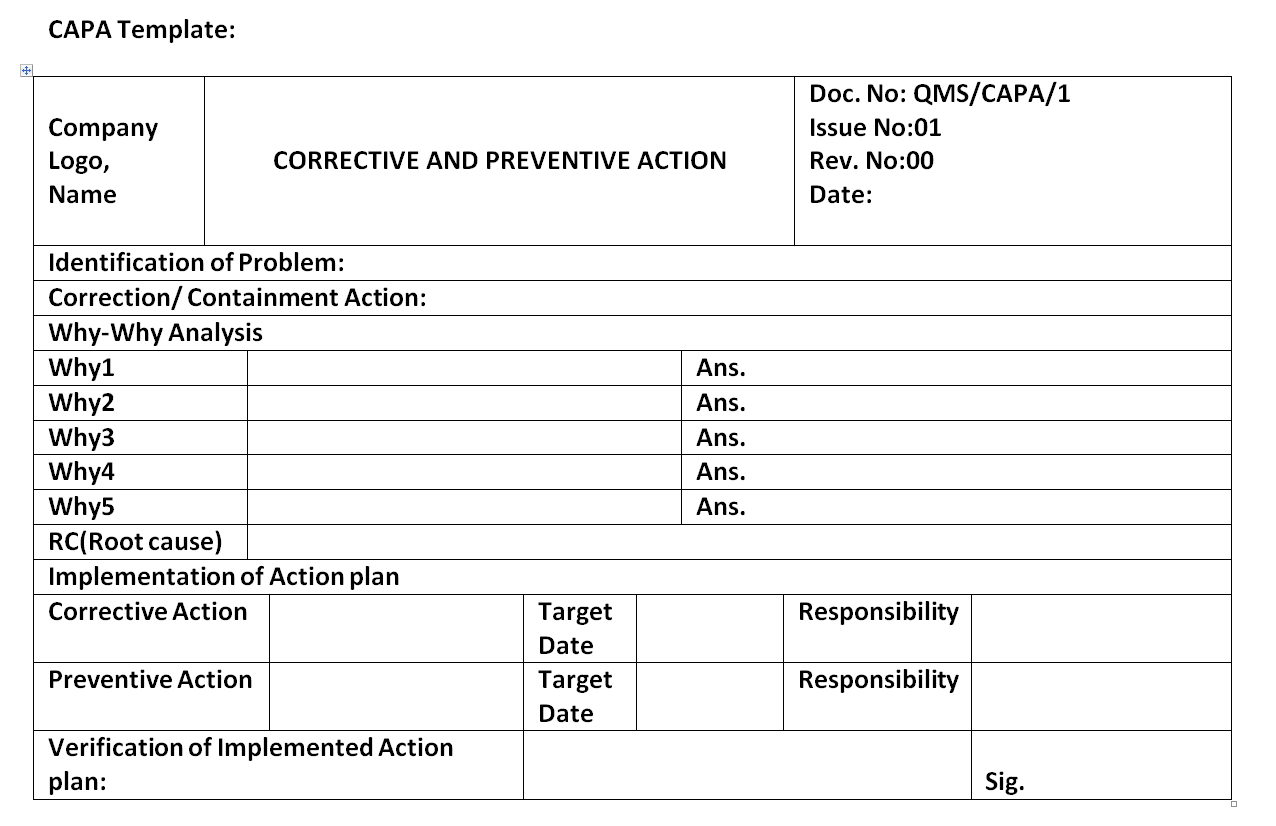
Corrective and Preventive Action Format CAPA with Example

How to implement Corrective action Preventive action?

Improving Processes and CAPA (Corrective and Preventative Action)
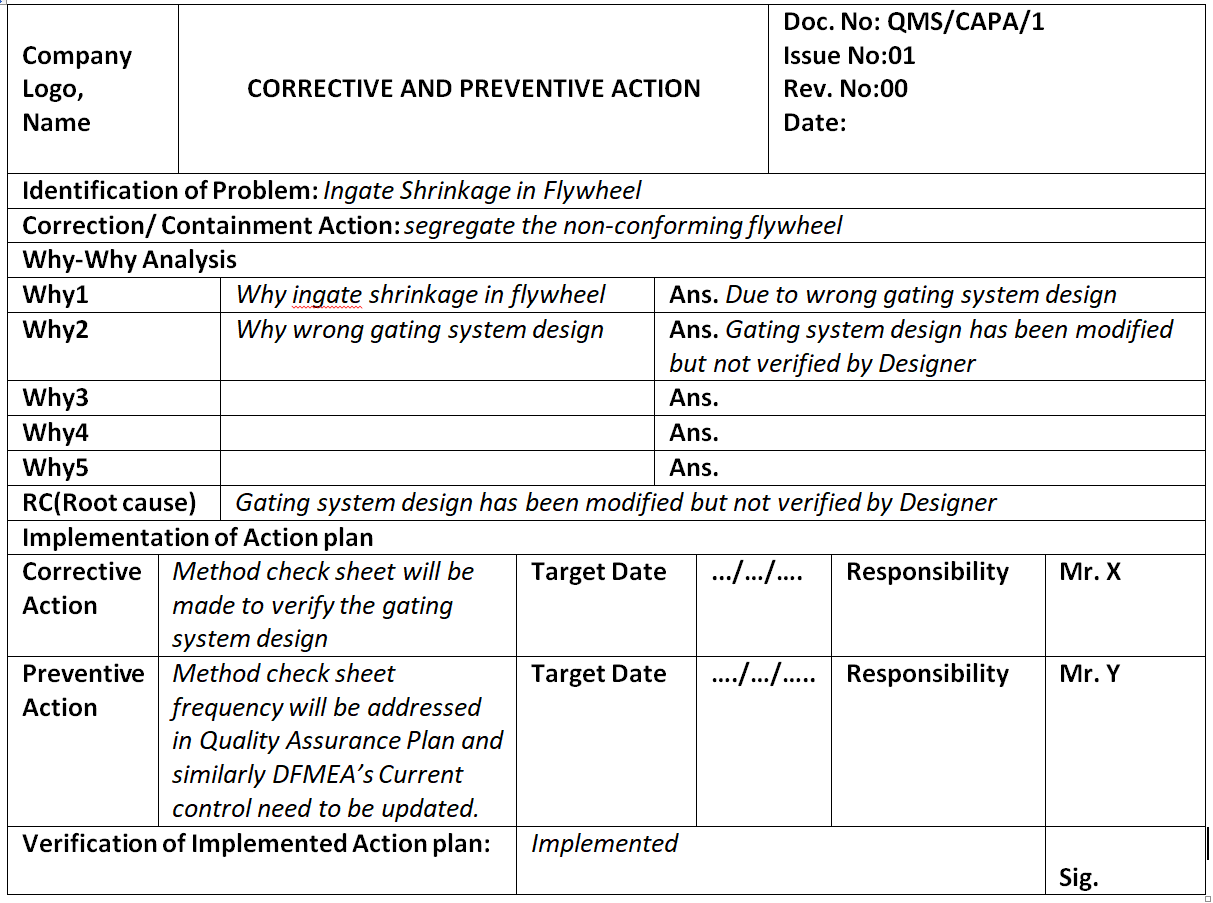
Corrective and Preventive Action Format CAPA with Example
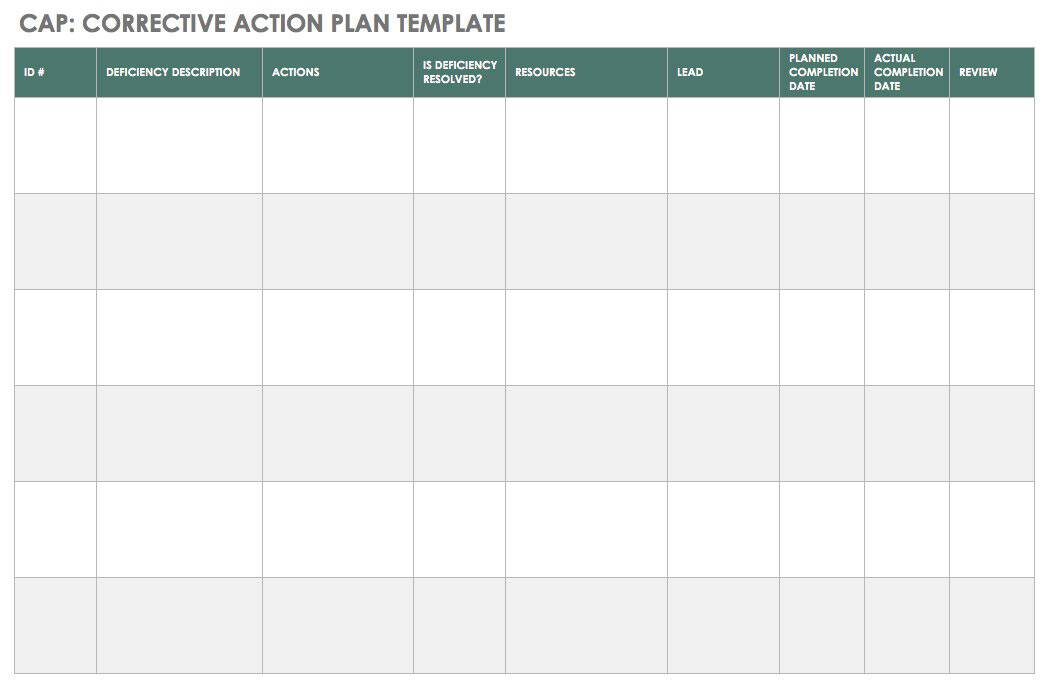
The Beginner’s Guide to CAPA Smartsheet
Web Download Corrective And Preventative Action Plan Form Template_2019.11.13.
Web Corrective And Preventive Action (Capa):
Corrective Action And Preventive Action (Capa) Plan Template.
Web This Is A Free Template, Provided By Openregulatory.
Related Post: