Capa Plan Template
Capa Plan Template - •define capa and state the purpose of capas •list components of a capa •what makes a successful capa •apply capa principles when preparing for. Web preventive action plan (capa) a corrective and preventive action plan (capa) will identify the source of a problem and take corrective measures to avoid recurrences. Create effective capa forms using a simple template. Capa reports are typically initiated in response to. Web click here, to access the corrective and preventive action plan template with sample data. Web using a corrective action template helps you create an official document that offers guidance in the process. Web corrective and preventive actions (capa) plans. There are different templates you can use including product. This template is designed to. Web capa should be signed by the author, submitted to the irb for review, kept on file in the site regulatory file. Follow the steps to conduct a root cause. Web capa should be signed by the author, submitted to the irb for review, kept on file in the site regulatory file. Corrective and preventive actions (capa) inspectional objectives. This template is designed to. •define capa and state the purpose of capas •list components of a capa •what makes a successful capa. Corrective and preventive actions (capa) inspectional objectives. Follow the steps to conduct a root cause. Web click here, to access the corrective and preventive action plan template with sample data. Web learn how to identify, evaluate, and respond to deviations and unexpected events in research to protect participants and data. Guiding clinical research professionals in improving weaknesses, deficiencies, or in. Web the iu hrpp quality improvement office (qio) has developed a capa plan template to assist study teams with this activity. Corrective action and preventive action (capa) plan template. Web free lean six sigma templates. Web learn how to identify, evaluate, and respond to deviations and unexpected events in research to protect participants and data. Web this capa report template. Create effective capa forms using a simple template. Corrective and preventive actions (capa) inspectional objectives. Web corrective and preventive actions (capa) plans. Web preventive action plan (capa) a corrective and preventive action plan (capa) will identify the source of a problem and take corrective measures to avoid recurrences. The following is a template for the content and format. Web learn how to identify, evaluate, and respond to deviations and unexpected events in research to protect participants and data. As testers, we contribute to the success of a. Staying on the journey to success: How capa can sustain your organization. Create effective capa forms using a simple template. Web instructions if you have been informed by the irb that a response to an audit is required please utilize this document to form a response to the audit findings please […] Guiding clinical research professionals in improving weaknesses, deficiencies, or in rectifying deviation patterns and areas of. This template is designed to. Web free lean six sigma templates. Follow. Corrective and preventive actions (capa) inspectional objectives. Web preventive action plan (capa) a corrective and preventive action plan (capa) will identify the source of a problem and take corrective measures to avoid recurrences. This template is designed to. Web capa should be signed by the author, submitted to the irb for review, kept on file in the site regulatory file.. Web this capa report template can be used by compliance officers when formulating a corrective action to resolve issues and discuss preventive actions to lower. Web free lean six sigma templates. Create effective capa forms using a simple template. There are different templates you can use including product. Web click here, to access the corrective and preventive action plan template. Web instructions if you have been informed by the irb that a response to an audit is required please utilize this document to form a response to the audit findings please […] Capa corrective action plans corrective. Guiding clinical research professionals in improving weaknesses, deficiencies, or in rectifying deviation patterns and areas of. Web preventive action plan (capa) a corrective. Follow the steps to conduct a root cause. As testers, we contribute to the success of a. Create effective capa forms using a simple template. Web click here, to access the corrective and preventive action plan template with sample data. How capa can sustain your organization. Web preventive action plan (capa) a corrective and preventive action plan (capa) will identify the source of a problem and take corrective measures to avoid recurrences. Corrective and preventive actions (capa) form a key approach in quality management systems. Capa corrective action plans corrective. There are different templates you can use including product. Web click here, to access the corrective and preventive action plan template with sample data. Web this capa report template can be used by compliance officers when formulating a corrective action to resolve issues and discuss preventive actions to lower. Corrective action and preventive action (capa) plan template. Staying on the journey to success: Web free lean six sigma templates. Follow the steps to conduct a root cause. Web corrective and preventive actions (capa) plans. Verification of corrective and preventive actions. Web learn how to identify, evaluate, and respond to deviations and unexpected events in research to protect participants and data. Guiding clinical research professionals in improving weaknesses, deficiencies, or in rectifying deviation patterns and areas of. As testers, we contribute to the success of a. •define capa and state the purpose of capas •list components of a capa •what makes a successful capa •apply capa principles when preparing for.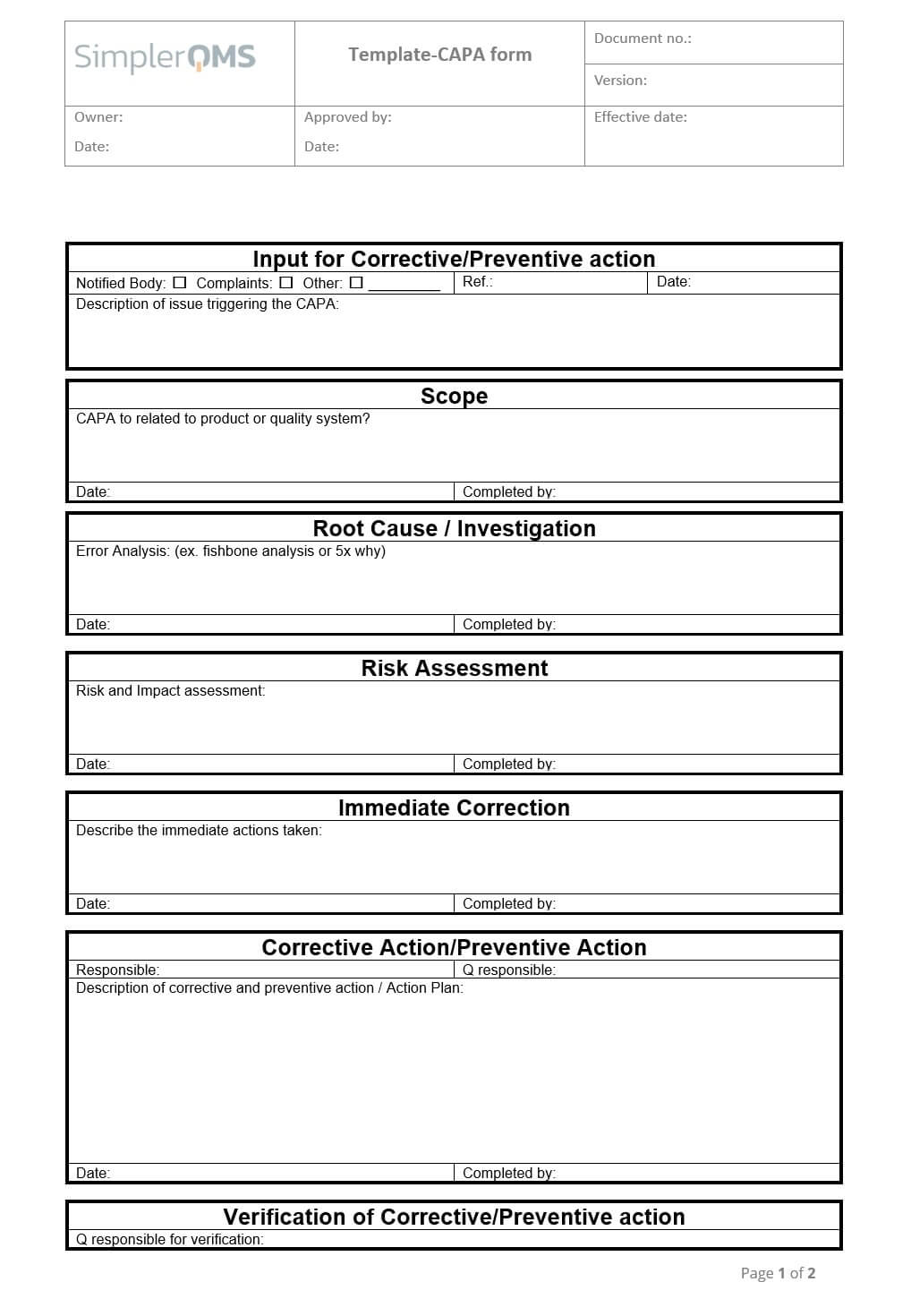
Corrective and Preventive Action (CAPA) Form Template SimplerQMS

SOP For Corrective Action and Preventive Actions Pharmaceutical
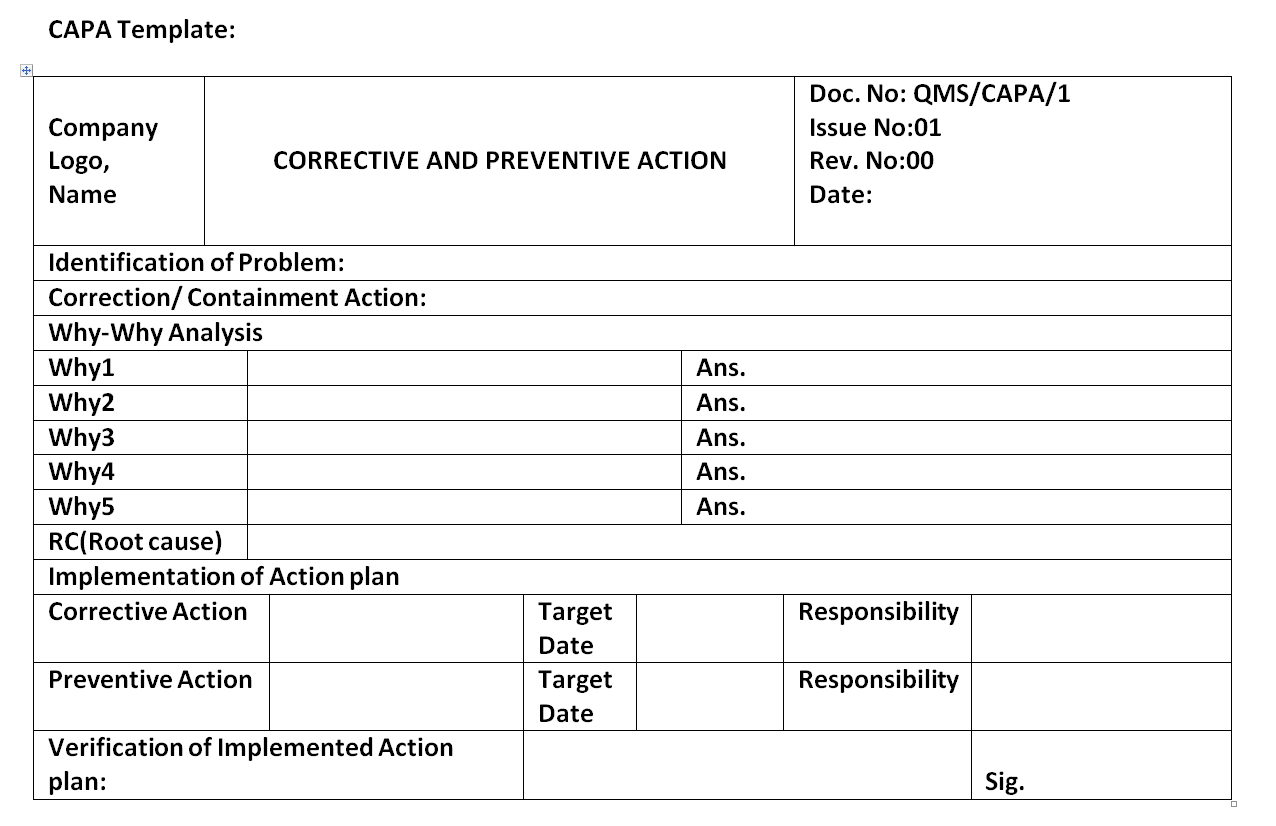
Corrective and Preventive Action Format CAPA with Example

Corrective and preventive action plan CAPA report form

How to implement Corrective action Preventive action?

Sample Capa form Beautiful Corrective Action Report Example Action
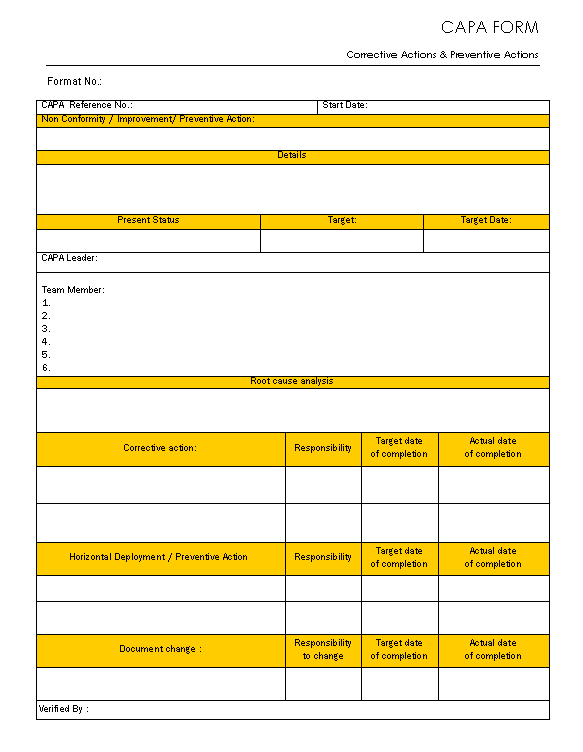
CAPA form Corrective action and preventive action
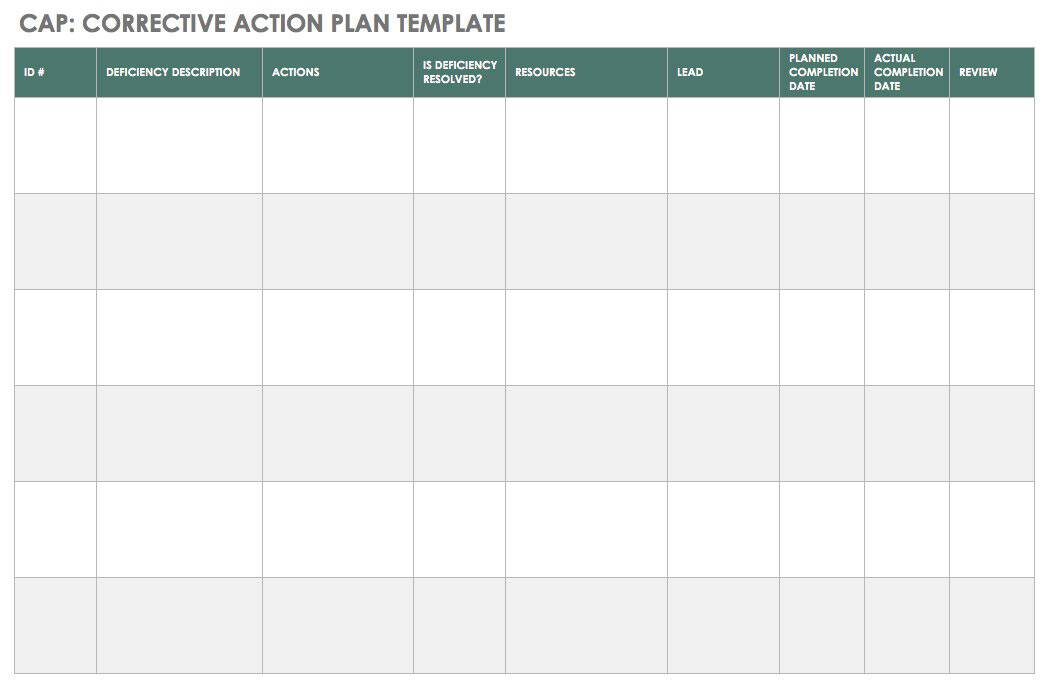
food and Health safety updates CAPA corrective action and preventive

Corrective and preventive action plan CAPA report form
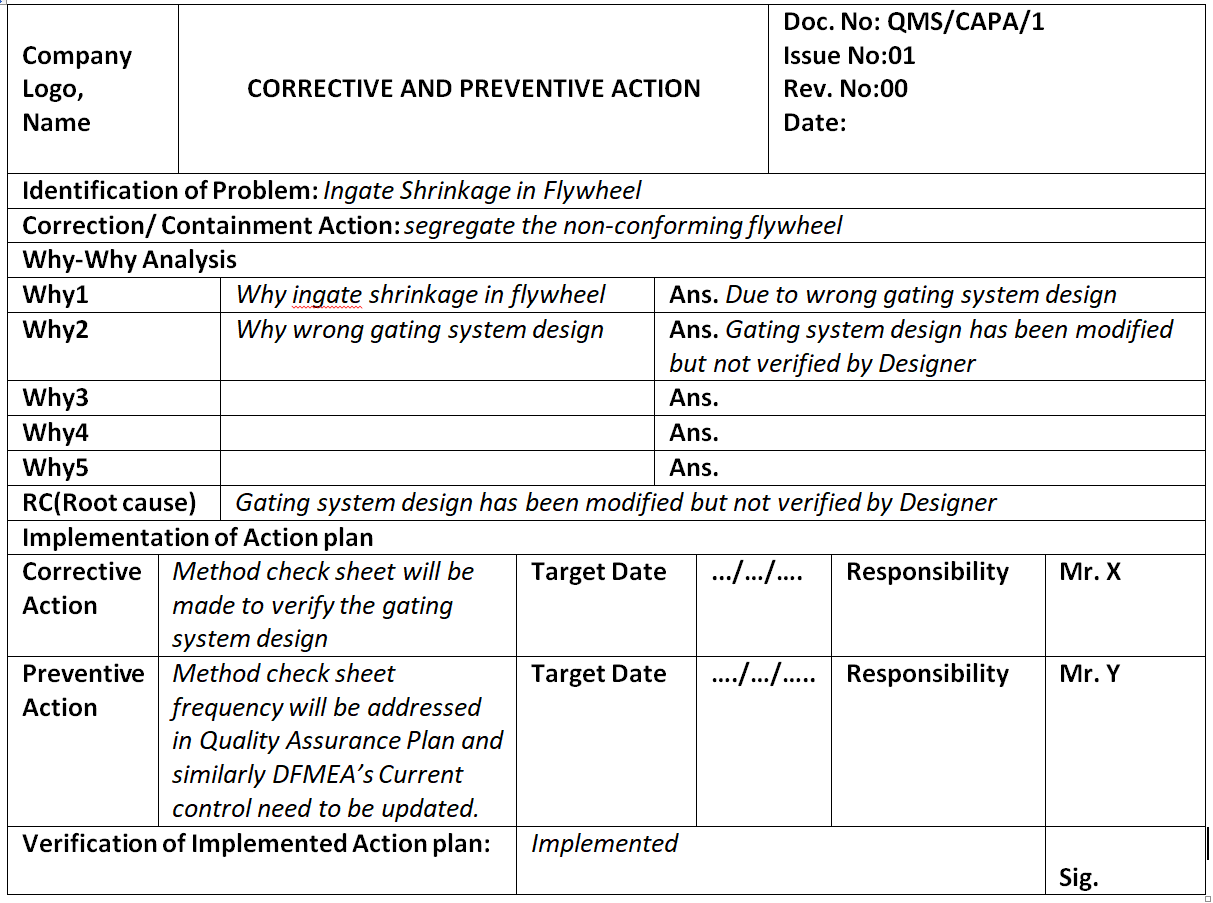
Corrective And Preventive Action Plan Template
Web Using A Corrective Action Template Helps You Create An Official Document That Offers Guidance In The Process.
Capa Reports Are Typically Initiated In Response To.
This Template Is Designed To.
Corrective And Preventive Actions (Capa) Inspectional Objectives.
Related Post: